The Effect of Nutritional Interventions on Long-Term Patient Survival in Advanced Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Nutrition Education Program
2.4. Laboratory Parameters
2.5. Anthropometrics and Body Composition
2.6. Dietary Intake
2.7. Nutritional Assessment
- Biochemical category: albumin <3.8 g/dL; prealbumin <30 mg/dL body mass; cholesterol <100 mg/dL without lipid-lowering medication (in our case, this criterion was not employed, given that 147 patients consumed such medication).
- Body mass category: BMI <23 kg/m2; unintentional 5% weight loss over the last 3 months or 10% weight loss over the last 6 months.
- Muscle mass category: loss of 10% of MAMC in relation to the 50th percentile.
- Intake category: protein catabolism rate (normalized protein nitrogen) <0.6 g/kg/day; energy intake <25 kcal/kg adjusted to weight/day.
2.8. Number of Hospital Admissions
2.9. Mortality
2.10. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
3.2. Results of the Nutritional Education Program at 6 Months
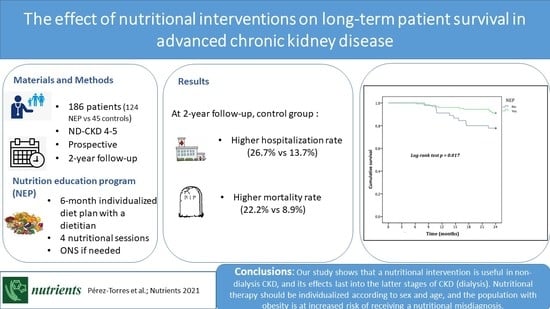
3.3. Comorbidities and Survival at the 2-Year Follow-Up
4. Discussion
4.1. Nutritional Education Program Efficacy at 6 Months
4.2. Long-Term Effects of Nutritional Education Programs on Health Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 286, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kopple, D.J.; Kalantar-Zadeh, K. Management of proteinenergy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clinnutr. 2013, 97, 1163–1177. [Google Scholar]
- Kovesdy, C.P.; George, S.M.; Anderson, J.E.; Kalantar-Zadeh, K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am. J. Clin. Nutr. 2009, 90, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Ash, S.; Davies, P.S.; Bauer, J.D. Randomized controlled trial of nutritional counselling on body composition and dietary intake in severe CKD. Am. J. Kidney. Dis. 2008, 51, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Cliffe, M.; Bloodworth, L.L.; Jibani, M.M. Can malnutrition in predialysis patients be prevented by dietetic intervention? J. Ren. Nutr. 2001, 11, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Molfino, A.; Coppola, B.; Deleo, S.; Tommasi, V.; Galani, A.; Migliaccio, S.; Greco, E.A.; Gnerremusto, T.; Muscaritoli, M. Effect of personalized dietary intervention on nutritional, metabolic and vascular indices in patients with chronic kidney disease. Eur. Rev. Med. Pharm. Sci. 2015, 19, 3351–3359. [Google Scholar]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37 (Suppl 2), S66–S70. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl 1), S1–S107. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 846, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Methodology of Nutritinal Surveillance; Report of Ajoint FAO/UNICEF/WHO Expert Consultation; WHO: Geneva, Switzerland, 1976. [Google Scholar]
- Jagadeswaran, D.; Indhumathi, E.; Hemamalini, A.J.; Sivakumar, V.; Soundararajan, P.; Jayakumar, M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin. Nutr. 2019, 381, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Jeloka, T.K.; Dharmatti, G.; Jamdade, T.; Pandit, M. Are oral protein supplements helpful in the management of malnutrition in dialysis patients? Indian J. Nephrol. 2013, 231, 1–4. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 77, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Piotti, G.; Cosola, C.; Gandolfini, I.; Kooman, J.P.; Fiaccadori, E. Dietary protein and nutritional supplements in conventional hemodialysis. Semin. Dial. 2018, 316, 583–591. [Google Scholar] [CrossRef]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-term oral nutrition supplementation improves outcomes in malnourished patients with chronic kidney disease on hemodialysis. JPEN J. Parenter. Enteral Nutr. 2014, 388, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Hernández Morante, J.J.; Sánchez-Villazala, A.; Cutillas, R.C.; Fuentes, M.C. Effectiveness of a nutrition education program for the prevention and treatment of malnutrition in end-stage renal disease. J. Ren. Nutr. 2014, 241, 42–49. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 183, 254–262. [Google Scholar] [CrossRef]
- Ruiz, A.J.; Buitrago, G.; Rodríguez, N.; Gómez, G.; Sulo, S.; Gómez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2019, 383, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Yárnoz-Esquíroz, P.; Lacasa, C.; Riestra, M.; Silva, C.; Frühbeck, G. Clinical and financial implications of hospital malnutrition in Spain. Eur. Eat. Disord. Rev. 2019, 276, 581–602. [Google Scholar] [CrossRef]
- Cano, N.J.M.; Fouque, D.; Roth, H.; Aparicio, M.; Azar, R.; Canaud, B.; Chauveau, P.; Combe, C.; Laville, M.; Leverve, X.M. Intradialytic Parenteral Nutrition Does Not Improve Survival in Malnourished Hemodialysis Patients: A 2-Year Multicenter, Prospective, Randomized Study. J. Am. Soc. Nephrol. 2007, 18, 2583–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazairac, A.H.; de Wit, G.A.; Grooteman, M.P.; Penne, E.L.; van der Weerd, N.C.; van den Dorpel, M.A.; Nubé, M.J.; Lévesque, R.; Ter Wee, P.M.; Bots, M.L.; et al. A composite score of protein-energy nutritional status predicts mortality in haemodialysis patients no better than its individual components. Nephrol. Dial. Transplant. 2011, 266, 1962–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, R.; Chiu, Y.W.; Kalantar-Zadeh, K.; Bargman, J.; Vonesh, E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch. Intern. Med. 2011, 1712, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaar, B.G.; Plantinga, L.C.; Crews, D.C.; Fink, N.E.; Hebah, N.; Coresh, J.; Kliger, A.S.; Powe, N.R. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: A prospective study. BMC Nephrol. 2009, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Sladoje-Martinovic, B.; Mikolasevic, I.; Bubic, I.; Racki, S.; Orlic, L. Survival of chronic hemodialysis patients over 80 years of age. Clin. Interv. Aging 2014, 9, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, A.; Markell, M. Impact of gender and gender disparities in patients with kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 282, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 143, 151–164. [Google Scholar] [CrossRef] [PubMed]



| NEP+ONS | NEP | |||||
|---|---|---|---|---|---|---|
| Month 0 | Month 6 | p | Month 0 | Month 6 | p | |
| Age | 70.5 ± 14.5 | 66.0 ± 14.8 | ||||
| Albumin, g/dL | 3.3 ± 0.5 * | 3.5 ± 0.5 | 0.106 | 3.6 ± 0.4 | 3.8 ± 0.3 | <0.05 |
| Prealbumin, mg/dL | 26.0 ± 5.6 * | 30.0 ± 4.4 b | <0.05 | 31.1 ± 6.4 | 32.1 ± 6.2 b | 0.480 |
| CrCl, ml/min | 16.8 ± 4.1 | 18.9 ± 6.2 | <0.05 | 17.6 ± 3.8 | 19.5 ± 6.5 | <0.05 |
| Creatinine, mg/dL | 3.4 ± 1.1 | 3.3 ± 1.0 | 0.252 | 3,8 ± 1,2 | 3.6 ± 1.3 | 0.400 |
| Proteinuria, g/24 | 2.0 ± 3.1 | 1.8 ± 2.8 | 0.086 | 1.7 ± 1.6 | 1.6 ± 1.7 | 0.176 |
| Potassium, meq/L | 4.8 ± 0.6 | 4.6 ± 0.5 | 0.105 | 4.8 ± 0.6 | 4.6 ± 0.5 | <0.05 |
| Phosphorous, mg/dL | 3.8 ± 0.9 | 3.8 ± 0.6 | 0.592 | 4.1 ± 0.9 | 3.9 ± 0.7 | <0.05 |
| LDL, mg/dL | 109.1 ± 29.2 | 106 ± 30.3 | 0.411 | 114.0 ± 35.2 | 104.9 ± 28.1 | <0.05 |
| Triglycerides, mg/dL | 125.3 ± 53.7 | 120.2 ± 48.4 | 0.362 | 141.5 ± 61.6 | 125.6 ± 38.8 | <0.05 |
| Weight, kg | 61.5 ± 13.1 * | 62.2 ± 11.3 b | 0.590 | 73.9 ± 12.4 | 72.2 ± 11.3 b | <0.001 |
| Height, m | 160.7 ± 9.8 | 159.1 ± 8.9 | ||||
| BMI, kg/m2 | 24.5 ± 5.4 * | 24.7 ± 4.6 b | <0.05 | 28.8 ± 4.4 | 28.0 ± 4.0 b | <0.001 |
| TSF, mm | 15.3 ± 7.1 * | 15.5 ± 7.1 b | 0.091 | 20.1 ± 7.1 | 19.7 ± 7.0 b | <0.001 |
| MAMC, mm2 | 20.5 ± 3.5 * | 21.0 ± 3.4 | <0.05 | 23.9 ± 3.9 | 23.9 ± 3.6 | 0.956 |
| Resistance, ohms | 550.4 ± 99.9 | 535.3 ± 96.9 b | 0.061 | 507.3 ± 74.7 | 506.4 ± 79.9 b | 0.073 |
| Reactance, ohms | 33.4 ± 7.7 | 41.7 ± 8.5 b | 0.053 | 44.8 ± 10.9 | 46.3 ± 10.7 b | 0.073 |
| Phase angle, degrees | 3.9 ± 0.9 | 4.5 ± 0.8 b | 0.051 | 5.2 ± 1.2 | 5.21 ± 0.6 b | 0.540 |
| Na/K Exchange | 1.6 ± 0.5 | 1.4 ± 0.5 b | 0.059 | 1.0 ± 0.2 | 1.0 ± 0.5 b | 0.974 |
| TBW, % | 57.7 ± 6.0 | 54.6 ± 9.6 b | 0.054 | 52.5 ± 6.1 | 52.6 ± 6.0 b | 0.608 |
| ECW, % | 58.7 ± 6.2 | 53.6 ± 4.4 b | <0.05 | 50.5 ± 5.8 | 49.5 ± 5.5 b | <0.05 |
| ICW, % | 41.1 ± 6.2 | 46.4 ± 4.4 b | <0.05 | 49.4 ± 5.8 | 50.6 ± 5.5 b | 0.070 |
| Fat mass, % | 27.7 ± 12.5 | 30.8 ± 11.9 b | <0.05 | 33.4 ± 8.4 | 32.7 ± 8.8 b | 0.634 |
| Muscle mass, % | 39 ± 13.6 | 40.2 ± 12.7 | 0.084 | 38.5 ± 7.6 | 40.4 ± 7.5 | <0.05 |
| Body cellular mass index | 7.0 ± 2.6 | 7.7 ± 2.7 | <0.05 | 8.2 ± 2.1 | 8.5 ± 1.8 | <0.05 |
| NEP group N = 124 (73.4%) | Control Group N = 45 (26.6%) | |||||
|---|---|---|---|---|---|---|
| Month 0 | 2 Years | Month 0 | 2 Years | |||
| Age, years | 67.2 ± 14.9 | 63.2±18.3 | 0.036 | |||
| Male sex, n (%) | 57 (46%) | 32 (71.1%) | 0.050 | |||
| Albumin, g/dL | 3.5 ± 0.4 | 3.8 ± 0.5 | <0.001 | 3.6±0.4 | 3.4 ± 0.5 | 0.027 |
| nPNA, g/kg/day | 1.18 ± 0.35 | 1.30 ± 0.27 | 0.020 | 1.2±0.35 | 1.3 ± 0.34 | 0.617 |
| Weight, kg | 70.5 ± 13.3 * | 70.1 ± 11.6 | 0.084 | 74.0±18.2* | 75.0 ± 20.6 | 0.635 |
| BMI, kg/m2 | 27.55 ± 4.9 | 27.5 ± 4.4 | 0.110 | 27.0±5.3 | 27.1 ± 6.18 | 0.635 |
| Multivariate Model | |||||
|---|---|---|---|---|---|
| N | OR | 95% CI | p | ||
| Age | 169 | 1.094 | 1.035–1.157 | 0.001 | |
| Sex | Male | 80 | |||
| Female | 89 | 3.332 | 1.054–10.535 | 0.040 | |
| NEP | Yes | 124 | |||
| No | 45 | 2.883 | 0.993–8.365 | 0.051 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Torres, A.; González García, M.E.; Ossorio-González, M.; Álvarez García, L.; Bajo, M.A.; del Peso, G.; Castillo Plaza, A.; Selgas, R. The Effect of Nutritional Interventions on Long-Term Patient Survival in Advanced Chronic Kidney Disease. Nutrients 2021, 13, 621. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020621
Pérez-Torres A, González García ME, Ossorio-González M, Álvarez García L, Bajo MA, del Peso G, Castillo Plaza A, Selgas R. The Effect of Nutritional Interventions on Long-Term Patient Survival in Advanced Chronic Kidney Disease. Nutrients. 2021; 13(2):621. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020621
Chicago/Turabian StylePérez-Torres, Almudena, M. Elena González García, Marta Ossorio-González, Laura Álvarez García, M. Auxiliadora Bajo, Gloria del Peso, Ana Castillo Plaza, and Rafael Selgas. 2021. "The Effect of Nutritional Interventions on Long-Term Patient Survival in Advanced Chronic Kidney Disease" Nutrients 13, no. 2: 621. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020621