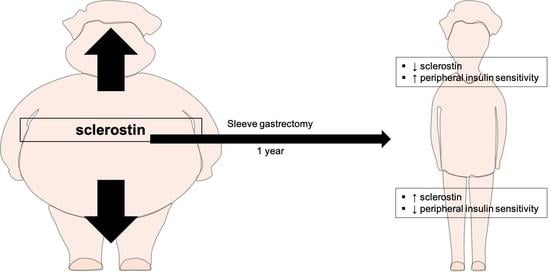
Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Anthropometric and Biochemical Assessment
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Metabolic Surgery Is Associated with an Increase in Circulating Levels of Sclerostin
3.2. Circulating Levels of Sclerostin Are Independently Correlated with Adipose Tissue Depots and Glycemic Profile at Baseline
3.3. Post-Surgical Variations in Serum Levels of Sclerostin Are Associated with Lean Mass Reduction and Insulin Resistance Improvement
3.4. Baseline Serum Levels of Sclerostin Are Negative Predictors of Worse Glycemic Recovery after SG.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pareek, M.; Schauer, P.R.; Kaplan, L.M.; Leiter, L.A.; Rubino, F.; Bhatt, D.L. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J. Am. Coll. Cardiol. 2018, 71, 670–687. [Google Scholar] [CrossRef]
- Carbone, F.; Nulli Migliola, E.; Bonaventura, A.; Vecchie, A.; De Vuono, S.; Ricci, M.A.; Vaudo, G.; Boni, M.; Dallegri, F.; Montecucco, F.; et al. High serum levels of C-reactive protein (CRP) predict beneficial decrease of visceral fat in obese females after sleeve gastrectomy. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C.A.; Umashanker, D.; Aronne, L.J.; Shukla, A.P. Intestinal and Gastric Origins for Diabetes Resolution After Bariatric Surgery. Curr. Obes. Rep. 2018, 7, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Epstein, S.; Napoli, N. Insulin resistance and bone: A biological partnership. Acta Diabetol. 2018, 55, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Roh, E.; Hong, S.H.; Lee, Y.B.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, N.H.; Kim, S.G.; Baik, S.H.; et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone 2019, 128, 115053. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Adami, G.; Liberale, L.; Bonaventura, A.; Bertolotto, M.; Andraghetti, G.; Scopinaro, N.; Camerini, G.B.; Papadia, F.S.; Cordera, R.; et al. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes. Metab. 2019, 45, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, D.; Czibik, G.; Pini, M.; Ternacle, J.; Suffee, N.; Mercedes, R.; Marcelin, G.; Surenaud, M.; Marcos, E.; Gual, P.; et al. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018, 138, 809–822. [Google Scholar] [CrossRef]
- Daniele, G.; Winnier, D.; Mari, A.; Bruder, J.; Fourcaudot, M.; Pengou, Z.; Tripathy, D.; Jenkinson, C.; Folli, F. Sclerostin and Insulin Resistance in Prediabetes: Evidence of a Cross Talk Between Bone and Glucose Metabolism. Diabetes Care 2015, 38, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Adami, G.F.; Carbone, F.; Montecucco, F.; Camerini, G.; Cordera, R. Adipose Tissue Composition in Obesity and After Bariatric Surgery. Obes. Surg. 2019, 29, 3030–3038. [Google Scholar] [CrossRef]
- Hirooka, M.; Kumagi, T.; Kurose, K.; Nakanishi, S.; Michitaka, K.; Matsuura, B.; Horiike, N.; Onji, M. A technique for the measurement of visceral fat by ultrasonography: Comparison of measurements by ultrasonography and computed tomography. Intern. Med. 2005, 44, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study, G. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Geloneze, B.; Vasques, A.C.; Stabe, C.F.; Pareja, J.C.; Rosado, L.E.; Queiroz, E.C.; Tambascia, M.A.; Investigators, B. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. DE Endocrinol. E Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Villarreal-Calderon, J.R.; Cuellar, R.X.; Ramos-Gonzalez, M.R.; Rubio-Infante, N.; Castillo, E.C.; Elizondo-Montemayor, L.; Garcia-Rivas, G. Interplay between the Adaptive Immune System and Insulin Resistance in Weight Loss Induced by Bariatric Surgery. Oxid. Med. Cell Longev. 2019, 2019, 3940739. [Google Scholar] [CrossRef] [Green Version]
- Bluher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. 2016, 130, 1603–1614. [Google Scholar] [CrossRef]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Vecchie, A.; Bonomi, A.; Scopinaro, N.; Camerini, G.B.; Papadia, F.S.; Maggi, D.; Cordera, R.; et al. Baseline neutrophil-to-lymphocyte ratio is associated with long-term T2D remission after metabolic surgery. Acta Diabetol. 2019, 56, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.H.; Richards, B.; Berger, C.; Josse, R.G.; Leslie, W.D.; Goltzman, D.; Kaiser, S.M.; Kovacs, C.S.; Davison, K.S. The association between sclerostin and incident type 2 diabetes risk: A cohort study. Clin. Endocrinol. 2017, 86, 520–525. [Google Scholar] [CrossRef]
- Ebenibo, S.E.C.; Dagogo-Jack, S. Plasma FGF-21 and sclerostin levels predict glycemia and insulin sensitivity in healthy subjects with parental type 2 diabetes. In Proceedings of the Determinants of Insulin Resistance & Associated Metabolic Disturbance session of Endo 2013, San Francisco, CA, USA, 15 January 2021. [Google Scholar]
- Kim, S.P.; Frey, J.L.; Li, Z.; Kushwaha, P.; Zoch, M.L.; Tomlinson, R.E.; Da, H.; Aja, S.; Noh, H.L.; Kim, J.K.; et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E11238–E11247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, L.G.M.; Van Dam, A.D.; Hanssen, M.J.W.; Kooijman, S.; Nahon, K.J.; Reinders, H.; Jazet, I.M.; Van Marken Lichtenbelt, W.D.; Rensen, P.C.N.; Appelman-Dijkstra, N.M.; et al. Higher Plasma Sclerostin and Lower Wnt Signaling Gene Expression in White Adipose Tissue of Prediabetic South Asian Men Compared with White Caucasian Men. Diabetes Metab. J. 2020, 44, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeve, D.; Seo, J.; Suh, J.M.; Stenesen, D.; Tang, W.; Berglund, E.D.; Wan, Y.; Williams, L.J.; Lim, A.; Martinez, M.J.; et al. Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab. 2012, 15, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; De Aguiar, R.B.; Naik, S.; Mani, S.; Ostadsharif, K.; Wencker, D.; Sotoudeh, M.; Malekzadeh, R.; Sherwin, R.S.; Mani, A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013, 17, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, S.C.N.; Wong, S.K.H.; Ai, Q.; Yeung, D.K.W.; Ng, E.K.W.; Chu, W.C.W. Observed changes in brown, white, hepatic and pancreatic fat after bariatric surgery: Evaluation with MRI. Eur. Radiol. 2019, 29, 849–856. [Google Scholar] [CrossRef]
- Lehmann, S.; Linder, N.; Retschlag, U.; Schaudinn, A.; Stange, R.; Garnov, N.; Dietrich, A.; Oberbach, A.; Kahn, T.; Busse, H. MRI assessment of changes in adipose tissue parameters after bariatric surgery. PLoS ONE 2018, 13, e0206735. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Gerspach, A.C.; Peterli, R.; Moor, M.; Madorin, P.; Schotzau, A.; Nabers, D.; Borgwardt, S.; Beglinger, C.; Bieri, O.; Wolnerhanssen, B.K. Quantification of Liver, Subcutaneous, and Visceral Adipose Tissues by MRI Before and After Bariatric Surgery. Obes. Surg. 2019, 29, 2795–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, G.; Castagneto Gissey, L.; Del Corpo, G.; Giordano, C.; Cerbelli, B.; Severino, A.; Manco, M.; Basso, N.; Birkenfeld, A.L.; Bornstein, S.R.; et al. New insight into the mechanisms of ectopic fat deposition improvement after bariatric surgery. Sci. Rep. 2019, 9, 17315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muschitz, C.; Kocijan, R.; Marterer, C.; Nia, A.R.; Muschitz, G.K.; Resch, H.; Pietschmann, P. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015, 100, 891–901. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [Green Version]
- Armamento-Villareal, R.; Sadler, C.; Napoli, N.; Shah, K.; Chode, S.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J. Bone. Miner. Res. 2012, 27, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.C.; Rocha, N.; Bandeira, E.; Dantas, I.; Chaves, C.; Oliveira, M.; Bandeira, F. Serum Sclerostin, Body Composition, and Sarcopenia in Hemodialysis Patients with Diabetes. Int. J. Nephrol. 2020, 2020, 4596920. [Google Scholar] [CrossRef] [Green Version]
- Vecchie, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Fruhbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic Obesity: Current Knowledge, Revised Identification Criteria and Treatment Principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.M.; Cruz-Jentoft, A.J.; Fielding, R.A.; Kanis, J.A.; Reginster, J.Y.; Bruyere, O.; Cesari, M.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; et al. Is There Enough Evidence for Osteosarcopenic Obesity as a Distinct Entity? A Critical Literature Review. Calcif. Tissue Int. 2019, 105, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofso, D.; Bollerslev, J.; Sandbu, R.; Jorgensen, A.; Godang, K.; Hjelmesaeth, J.; Ueland, T. Bone resorption following weight loss surgery is associated with treatment procedure and changes in secreted Wnt antagonists. Endocrine 2016, 53, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.P.; Fuleihan, G.E.-H. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos. Int. 2014, 25, 423–439. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, F.; Nulli Migliola, E.; Bonaventura, A.; Vecchié, A.; De Vuono, S.; Ricci, M.A.; Vaudo, G.; Boni, M.; Ministrini, S.; Lupattelli, G.; et al. Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy. Nutrients 2021, 13, 623. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020623
Carbone F, Nulli Migliola E, Bonaventura A, Vecchié A, De Vuono S, Ricci MA, Vaudo G, Boni M, Ministrini S, Lupattelli G, et al. Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy. Nutrients. 2021; 13(2):623. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020623
Chicago/Turabian StyleCarbone, Federico, Elisa Nulli Migliola, Aldo Bonaventura, Alessandra Vecchié, Stefano De Vuono, Maria Anastasia Ricci, Gaetano Vaudo, Marcello Boni, Stefano Ministrini, Graziana Lupattelli, and et al. 2021. "Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy" Nutrients 13, no. 2: 623. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020623