Influence of Micronutrient Intake, Sociodemographic, and Behavioral Factors on Periodontal Status of Adults Assisted by a Public Health Care System in Brazil: A Cross-Sectional Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Data Collection
2.3. Statistics
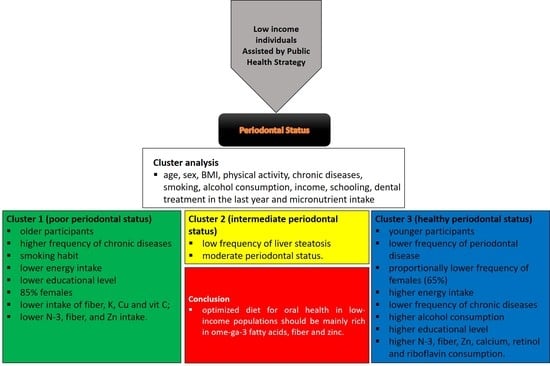
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.-P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus Report: Chronic Periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- Kinane, D.F.; Lappin, D.F. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol. Scand. 2001, 59, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rettori, E.; De Laurentiis, A.; Zubilete, M.Z.; Rettori, V.; Elverdin, J.C. Anti-Inflammatory Effect of the Endocannabinoid Anandamide in Experimental Periodontitis and Stress in the Rat. Neuroimmunomodulation 2012, 19, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Thrane, P.S.; Murison, R.; Gjermo, P. Emotional stress effects on immunity, gingivitis and periodontitis. Eur. J. Oral Sci. 1996, 104, 327–334. [Google Scholar] [CrossRef]
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, V.; Botelho, J.; Amaral, A.; Proença, L.; Alves, R.; Rua, J.; Cavacas, M.A.; Delgado, A.S.; Mendes, J.J. Prevalence and extent of chronic periodontitis and its risk factors in a Portuguese subpopulation: A retrospective cross-sectional study and analysis of Clinical Attachment Loss. PeerJ 2018, 6, e5258. [Google Scholar] [CrossRef]
- Sun, H.Y.; Jiang, H.; Du, M.Q.; Wang, X.; Feng, X.P.; Hu, D.Y.; Lin, H.C.; Wang, B.; Si, Y.; Wang, C.X.; et al. The Prevalence and Associated Factors of Periodontal Disease among 35 to 44-year-old Chinese Adults in the 4th National Oral Health Survey. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. (CSA) 2018, 21, 241–247. [Google Scholar]
- Pereira, L.J.; Gazolla, C.M.; Magalhães, I.B.; Dominguete, M.H.L.; Vilela, G.R.; Castelo, P.M.; Marques, L.S.; Van Der Bilt, A. Influence of periodontal treatment on objective measurement of masticatory performance. J. Oral Sci. 2012, 54, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingström, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, B.; Prasad, B.R.; Kumari, N.S.; Radhakrishna, V.; Ramesh, A. A comparative evaluation of the micronutrient profile in the serum of diabetes mellitus Type II patients and healthy individuals with periodontitis. J. Indian Soc. Periodontol. 2019, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.; Hasturk, H.; Mu, L.; Phillips, R.; Davis, R.; Halem, S.; Campos, H.; Goodson, J.; Van Dyke, T.; Mukamal, K. Docosahexaenoic Acid and Periodontitis in Adults. J. Dent. Res. 2014, 93, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.D.O.; Pereira, L.J.; Murata, R.M. Oral microbe-host interactions: Influence of β-glucans on gene expression of inflammatory cytokines and metabolome profile. BMC Microbiol. 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher Intakes of Fruits and Vegetables, β-Carotene, Vitamin C, α-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Shin, M.-S.; Kim, E.-J.; Ahn, Y.-B.; Kim, H.-D. The association of dietary vitamin C intake with periodontitis among Korean adults: Results from KNHANES IV. PLoS ONE 2017, 12, e0177074. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Giudice, A.L. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.; Araujo, J.; Barbosa, J. Decentralising the health sector: Issues in Brazil. Heal. Policy 2000, 52, 113–127. [Google Scholar] [CrossRef]
- Paim, J.; Travassos, C.; Almeida, C.; Bahia, L.; Macinko, J. The Brazilian health system: History, advances, and challenges. Lancet 2011, 377, 1778–1797. [Google Scholar] [CrossRef]
- Bastos, J.L.; Boing, A.F.; Peres, K.G.; Antunes, J.L.F.; Peres, M.A. Periodontal outcomes and social, racial and gender inequalities in Brazil: A systematic review of the literature between 1999 and 2008. Cad. de Saúde Pública 2011, 27, s141–s153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Ministry. Secretariat of Primary Health Care (SAPS). Family Health Strategy (FHS). 2019. Available online: https://aps.saude.gov.br/ape/esf/ (accessed on 12 March 2021).
- Mullachery, P.; Silver, D.; Macinko, J. Changes in health care inequity in Brazil between 2008 and 2013. Int. J. Equity Health 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Health Ministry. Secretariat of Primary Health Care (SAPS). e-Gestor Primary Care—Space for information and access to Primary Care systems. 2017. Available online: https://egestorab.saude.gov.br/ (accessed on 12 March 2021).
- Malta, D.C.; Santos, M.A.S.; Stopa, S.R.; Vieira, J.E.B.; Melo, E.A.; Dos Reis, A.A.C. A Cobertura da Estratégia de Saúde da Família (ESF) no Brasil, segundo a Pesquisa Nacional de Saúde, 2013. Ciência Saúde Coletiva 2016, 21, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morosini, M.V.; Fonseca, A.F. Community workers in Primary Health Care in Brazil: An inventory of achievements and challenges. Saúde Debate São Paulo 2018, 42, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Haas, A.; Wagner, M.C.; Oppermann, R.V.; Rösing, C.K.; Albandar, J.M.; Susin, C. Risk factors for the progression of periodontal attachment loss: A 5-year population-based study in South Brazil. J. Clin. Periodontol. 2014, 41, 215–223. [Google Scholar] [CrossRef]
- Bernal, R.T.I.; Iser, B.P.M.; Malta, D.C.; Claro, R.M. Sistema de Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (Vigitel): Mudança na metodologia de ponderação. Epidemiol. Serv. Saude Rev. Sist. Unico Saude Bras. 2017, 26, 701–712. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Colucci, A.C.A.; Morimoto, J.M.; Marchioni, D.M.L. Questionário de freqüência alimentar para adultos com base em estudo populacional. Rev. Saúde Pública 2008, 42, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Health Ministry. Orientações Para Coleta e Análise de Dados Antropométricos em Serviços de Saúde-Norma Técnica de Sistema de Vigilância Alimentar e Nutricional-SISVAN. 2011. Available online: https://sisaps.saude.gov.br/sisvan/ (accessed on 12 March 2021).
- Landry, R.; Jean, M. Periodontal Screening and Recording (PSR) Index: Precursors, utility and limitations in a clinical setting. Int. Dent. J. 2002, 52, 35–40. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Health Report 1995-Bridging the Gaps. 1995. Available online: https://www.who.int/whr/1995/en/ (accessed on 14 December 2020).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. WHO Expert Consultation; Technical Report Series No. 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Polk, D.E.; Wang, X.; Feingold, E.; Shaffer, J.R.; Weeks, D.E.; Weyant, R.J.; Crout, R.J.; McNeil, D.W.; Marazita, M.L. Effects of Smoking and Genotype on the PSR Index of Periodontal Disease in Adults Aged 18–49. Int. J. Environ. Res. Public Health 2012, 9, 2839–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padovani, R.M.; Amaya-Farfán, J.; Colugnati, F.A.B.; Domene, S.M. Álvares Dietary reference intakes: Aplicabilidade das tabelas em estudos nutricionais. Rev. Nutr. 2006, 19, 741–760. [Google Scholar] [CrossRef] [Green Version]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, G.; Ramakrishnan, T.; Parthasarathy, H.; Moses, J.; Lalitha, T. Evaluation of micronutrient (Zinc, Magnesium, and Copper) levels in serum and glycemic status after nonsurgical periodontal therapy in type 2 diabetic patients with chronic periodontitis. Contemp. Clin. Dent. 2017, 8, 26–32. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Beyersmann, D. Homeostasis and Cellular Functions of Zinc. Mater. Werkst. 2002, 33, 764–769. [Google Scholar] [CrossRef]
- Debjit Bhowmik, C.; Kumar, K.P.S. A potential medicinal importance of zinc in human health and chronic disease. Int. J. Pharm. Biomed. Sci. 2010, 1, 5–11. [Google Scholar]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Shan, Z.; Bao, W.; Zhang, Y.; Rong, Y.; Wang, X.; Jin, Y.; Song, Y.; Yao, P.; Sun, C.; Hu, F.B.; et al. Interactions Between Zinc Transporter-8 Gene (SLC30A8) and Plasma Zinc Concentrations for Impaired Glucose Regulation and Type 2 Diabetes. Diabetes 2013, 63, 1796–1803. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, G.B.; Brandão-Lima, P.N.; Maia, C.S.C.; Barbosa, K.B.F.; Pires, L.V. Zinc’s role in the glycemic control of patients with type 2 diabetes: A systematic review. BioMetals 2017, 30, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Saharia, G.K.; Goswami, R.K. Evaluation of Serum Zinc Status and Glycated Hemoglobin of Type 2 Diabetes Mellitus Patients in a Tertiary Care Hospital of Assam. J. Lab. Physicians 2013, 5, 30–33. [Google Scholar] [CrossRef]
- Sinha, S.; Sen, S. Status of zinc and magnesium levels in type 2 diabetes mellitus and its relationship with glycemic status. Int. J. Diabetes Dev. Ctries. 2014, 34, 220–223. [Google Scholar] [CrossRef]
- Andrade, E.F.; Lima, A.R.V.; Nunes, I.E.; Orlando, D.R.; Gondim, P.N.; Zangeronimo, M.G.; Alves, F.H.F.; Pereira, L.J. Exercise and Beta-Glucan Consumption (Saccharomyces cerevisiae) Improve the Metabolic Profile and Reduce the Atherogenic Index in Type 2 Diabetic Rats (HFD/STZ). Nutrients 2016, 8, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.D.O.; Lobato, R.V.; Andrade, E.F.; De Macedo, C.G.; Napimoga, J.T.C.; Napimoga, M.H.; Messora, M.R.; Murata, R.M.; Pereira, L.J. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PLoS ONE 2015, 10, e0134742. [Google Scholar] [CrossRef]
- Silva, V.D.O.; Lobato, R.V.; Andrade, E.F.; Orlando, D.R.; Borges, B.D.; Zangeronimo, M.G.; De Sousa, R.V.; Pereira, L.J. Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes. Nutrients 2017, 9, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobato, R.V.; Silva, V.D.O.; Andrade, E.F.; Orlando, D.R.; Zangeronimo, M.G.; De Sousa, R.V.; Pereira, L.J. Metabolic effects of-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 2015, 32, 256–264. [Google Scholar]
- Nielsen, S.J.; Trak-Fellermeier, M.A.; Joshipura, K.; Dye, B.A. Dietary Fiber Intake Is Inversely Associated with Periodontal Disease among US Adults. J. Nutr. 2016, 146, 2530–2536. [Google Scholar] [CrossRef] [Green Version]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Buttriss, J.L.; Stokes, C.S. Dietary fibre and health: An overview. Nutr. Bull. 2008, 33, 186–200. [Google Scholar] [CrossRef]
- Salvi, G.; Lang, N.P. The Effects of Non-Steroidal Anti-Inflammatory Drugs (Selective and Non-Selective) on the Treatment of Periodontal Diseases. Curr. Pharm. Des. 2005, 11, 1757–1769. [Google Scholar] [CrossRef]
- Azzi, D.V.; Viafara, J.A.S.; Zangeronimo, M.G.; Lima, R.R.; Marques, L.S.; Pereira, L.J. n-3 Ingestion may modulate the severity of periodontal disease? Systematic review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef]
- Gyurko, R.; Van Dyke, T.E. The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit. Rev. Immunol. 2014, 34, 347–357. [Google Scholar] [CrossRef]
- Balta, M.G.; Loos, B.G.; Nicu, E.A. Emerging Concepts in the Resolution of Periodontal Inflammation: A Role for Resolvin E1. Front. Immunol. 2017, 8, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, M.; Zhang, K.; Wei, Y.; Hua, W.; Gao, Y.; Li, X.; Ye, L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2019, 53, e12735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, N.C.C.; Andere, N.M.R.B.; Araujo, C.F.; De Marco, A.C.; Kantarci, A.; Van Dyke, T.E.; Santamaria, M.P. Omega-3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus: Randomized clinical trial. J. Periodontol. 2020, 91, 1318–1327. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Dixon, D.; Hildebolt, C.F.; Miley, D.D.; Garcia, M.N.; Pilgram, T.K.; Couture, R.; Spearie, C.A.; Civitelli, R. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br. Dent. J. 2009, 206, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Miley, D.D.; Garcia, M.N.; Hildebolt, C.F.; Shannon, W.D.; Couture, R.A.; Spearie, C.L.A.; Dixon, D.A.; Langenwalter, E.M.; Mueller, C.; Civitelli, R. Cross-Sectional Study of Vitamin D and Calcium Supplementation Effects on Chronic Periodontitis. J. Periodontol. 2009, 80, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Yamori, M.; Njelekela, M.; Mtabaji, J.; Yamori, Y.; Bessho, K. Hypertension, Periodontal Disease, and Potassium Intake in Nonsmoking, Nondrinker African Women on No Medication. Int. J. Hypertens. 2011, 2011, 695719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebersole, J.L.; Dawson, D.A.; Huja, P.E.; Pandruvada, S.; Basu, A.; Nguyen, L.; Zhang, Y.; Gonzalez, O.A. Age and Periodontal Health—Immunological View. Curr. Oral Health Rep. 2018, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Chatterjee, E.; Rajesh, K.S.; Kumar, M.S.A. Obesity and its association with chronic periodontitis: A cross-sectional study. J. Educ. Health Promot. 2019, 8, 222. [Google Scholar] [PubMed]
- Fentoğlu, Ö.; Öz, G.; Taşdelen, P.; Uskun, E.; Aykaç, Y.; Bozkurt, F.Y. Periodontal Status in Subjects with Hyperlipidemia. J. Periodontol. 2009, 80, 267–273. [Google Scholar] [CrossRef]
- Dutt, P.; Chaudhary, S.; Kumar, P. Oral Health and menopause: A comprehensive review on current knowledge and associated dental management. Ann. Med Health Sci. Res. 2013, 3, 320–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buencamino, M.C.A.; Palomo, L.; Thacker, H.L. How menopause affects oral health, and what we can do about it. Clevel. Clin. J. Med. 2009, 76, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ayed, M.S.; Alsharif, A.F.; Divakar, D.D.; Jhugroo, C.; Alosaimi, B.; Mustafa, M. Evaluating the possible association between systemic osteoporosis and periodontal disease progression in postmenopausal women. Disease-A-Month 2019, 65, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Miller, C.S.; Dawson, D.; Al-Sabbagh, M.; Ebersole, J.L. Cross-talk between clinical and host-response parameters of periodontitis in smokers. J. Periodontal Res. 2016, 52, 342–352. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Al-Sabbagh, M.; Gonzalez, O.A.; Dawson, D.R. Ageing effects on humoral immune responses in chronic periodontitis. J. Clin. Periodontol. 2018, 45, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Han, M.-L.; Teng, N.-C.; Lee, C.-Y.; Huang, W.-T.; Lin, C.-T.; Huang, Y.-K. Cigarette Smoking Aggravates the Activity of Periodontal Disease by Disrupting Redox Homeostasis—An Observational Study. Sci. Rep. 2018, 8, 11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Teughels, W.; on behalf of Group A of the European Workshop on Periodontology. Innovations in non-surgical periodontal therapy: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 3–7. [Google Scholar] [CrossRef]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette Smoking and Inflammation. J. Dent. Res. 2011, 91, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Choi, Y.-H.; Sagong, J.; Yu, S.; Kim, Y.; Lee, D.; Kim, S. The interactive association of smoking and drinking levels with presence of periodontitis in South Korean adults. BMC Oral Health 2016, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Britton, A.; Ben-Shlomo, Y.; Benzeval, M.; Kuh, D.; Bell, S. Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Giacosa, A.; Adam-Blondon, A.F.; Baer-Sinnott, S.; Barale, R.; Bavaresco, L.; Di Gaspero, G.; Dugo, L.; Ellison, R.C.; Gerbi, V.; Gifford, D.; et al. Alcohol and wine in relation to cancer and other diseases. Eur. J. Cancer Prev. 2012, 21, 103–108. [Google Scholar] [CrossRef]
- Szabo, G. Consequences of alcohol consumption on host defence. Alcohol 1999, 34, 830–841. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Wang, W.; Jiang, X. Alcohol consumption and risk of periodontitis: A meta-analysis. J. Clin. Periodontol. 2016, 43, 572–583. [Google Scholar] [CrossRef]
- Ministério da Saúde. (SAPS) Sistema de Informação e Gestão da Atenção Básica (e-Gestor). Available online: https://egestorab.saude.gov.br/index.xhtml (accessed on 2 August 2020).
- Pinto, L.F.; Giovanella, L. Do Programa à Estratégia Saúde da Família: Expansão do acesso e redução das internações por condições sensíveis à atenção básica (ICSAB). Ciência Saúde Coletiva 2018, 23, 1903–1914. [Google Scholar] [CrossRef]
- Cabral, J.F.; Da Silva, A.M.C.; Mattos, I.E.; Neves, Á.D.Q.; Luz, L.L.; Ferreira, D.B.; Santiago, L.M.; Carmo, C.N.D. Vulnerabilidade e fatores associados em idosos atendidos pela Estratégia Saúde da Família. Ciência Saúde Coletiva 2019, 24, 3227–3236. [Google Scholar] [CrossRef]
- Darmon, N.; Drewnowski, A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: A systematic review and analysis. Nutr. Rev. 2015, 73, 643–660. [Google Scholar] [CrossRef]
- Verly, E.; Darmon, N.; Sichieri, R.; Sarti, F.M. Reaching culturally acceptable and adequate diets at the lowest cost increment according to income level in Brazilian households. PLoS ONE 2020, 15, e0229439. [Google Scholar] [CrossRef] [PubMed]
- Macinko, J.; De Andrade, F.B.; Junior, P.R.B.D.S.; Lima-Costa, M.F.; De Souza, P.R.B. Primary care and healthcare utilization among older Brazilians (ELSI-Brazil). Rev. Saúde Pública 2018, 52, 6s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, M.F.D.L.; Modena, K.C.D.S.; Bresciani, E. Social disparity and oral health. Braz. Oral Res. 2012, 26, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, A.C.; Moysés, S.T.; Werneck, R.I.; Moysés, S.J. Oral health in the context of primary care in Brazil. Int. Dent. J. 2013, 63, 237–243. [Google Scholar] [CrossRef]


| Continuous Variables | Female Mean (±SD) | Male Mean (±SD) | Range (Female/Male) |
|---|---|---|---|
| Age (years) | 50 (12.5) | 51.9 (13.6) | 19–79/18–78 |
| BMI | 29.5 (6.1) | 27.6 (5.1) | 17–54.3/17.2–42.3 |
| Energy intake (Kcal/day) | 1495 (411.3) | 1756 (526.3) | 620–3214/772–3070 |
| Carbohydrates (g/day) | 219.26 (64.7) | 248.6 (75) | 89.6–475.8/108.5–437.9 |
| Lipids (g/day) | 41.6 (15.04) | 48.5 (18) | 12.5–92.1/10.6–102.1 |
| Protein (g/day) | 60.76 (18.5) | 73.8 (25.7) | 21.1–123.6/21.1–174 |
| Zinc (mg/day) | 7.41 (3.00) | 9.75 (5.52) | 1.9–19.6/2.4–36.5 |
| Fiber (g/day) | 21 (7.90) | 25 (9.47) | 3.4–46.8/7.6–44.8 |
| Omega–3 (mg/day) | 0.57 (0.21) | 0.69 (0.24) | 0.12–1.35/0.24–1.5 |
| Cholesterol (mg/day) | 229.5 (115.2) | 278.0 (169.9) | 15.5–972.1/72,6–1511.8 |
| Calcium (mg/day) | 418.3 (236.7) | 445.83 (268.29) | 52.2–1341.8/136.0–1295.5 |
| Magnesium (mg/day) | 195.79 (58.74) | 227.33 (76.83) | 66.0–402.7/77.30–451.9 |
| Manganese (mg/day) | 1.93 (0.60) | 2.22 (0.76) | 0.7–3.9/1.0–4.3 |
| Phosphorus (mg/day) | 892.69 (317.8) | 1056.29 (395.5) | 254.3–2142.8/367.2–2580.1 |
| Iron (mg/day) | 5.76 (1.89) | 6.83 (2.22) | 2.0–14.0/2.7–14.8 |
| Sodium (mg/day) | 1165.21 (473.16) | 1334.16 (487.9) | 287.3–3634.0/249.6–2902.9 |
| Potassium (mg/day) | 2193.11 (657.48) | 2407.46 (787.8) | 780.7–4686.0/850.8–4956.5 |
| Copper (mg/day) | 0.84 (0.44) | 0.86 (0.3) | 0.2–3.1/0.3–1.6 |
| Retinol (mg/day) | 170.30 (117.15) | 182.02 (121.92) | 4.2–779.8/19.70–654.80 |
| Thiamine (mg/day) | 0.82 (0.29) | 0.92 (0.35) | 0.2–2.0/0.3–2.4 |
| Riboflavin (mg/day) | 1.01 (0.52) | 1.06 (0.34) | 0.1–3.9/0.3–2.8 |
| Pyridoxine (mg/day) | 0.54 (0.31) | 0.70 (0.50) | 0.1–2.7/0–2.5 |
| Niacin (mg/day) | 12.56 (5.8) | 16.89 (10.15) | 1.6–45.1/2.40–58.90 |
| Vitamin C (mg/day) | 136.19 (101.63) | 123.06 (101.46) | 4.0–573.2/12.50–561.80 |
| Dichotomous variables | Female n (%) | Male n (%) | |
| Presence of at least one periodontal pocket ≥ 4 mm) | 234 (65.9%) | 70 (73.7%) | |
| Physical activity practice (≥ 3×/week) | 113 (32.8%) | 33 (34.7%) | |
| Diabetes mellitus (yes) | 100 (28%) | 27 (28.4%) | |
| Hypertension (yes) | 180 (50.7%) | 47 (49.5%) | |
| Hypercholesterolemia (yes) | 90 (25.4%) | 16 (16.8%) | |
| Hypertriglyceridemia (yes) | 7 (2%) | 0 | |
| Hypothyroidism (yes) | 26 (7.3%) | 2 (2.1%) | |
| Liver steatosis (yes) | 7 (2%) | 1 (1.1%) | |
| Cardiopathy (yes) | 22 (6.2%) | 3 (3.2%) | |
| Cancer history (yes) | 8 (2.3%) | 2 (2.1%) | |
| Depression (yes) | 24 (6.8%) | 3 (3.2%) | |
| Smoking (yes) | 74 (20.8%) | 26 (27.4%) | |
| Family income (>2 wages) | 120 (33.8%) | 33 (34.7%) | |
| Educational level (>8 years) | 194 (54.6%) | 50 (52.6%) | |
| Dental treatment last year | 123 (34.6%) | 43 (45.3%) | |
| Alcohol consumption (>2×/week) | 29 (8.2%) | 19 (20%) |
| Z Scores | Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Zscore (Omega-3) | 0.869 | 0.372 | |
| Zscore (total cholesterol) | 0.701 | 0.525 | |
| Zscore (fiber) | 0.819 | −0.588 | |
| Zscore (calcium) | 0.400 | −0.402 | 0.901 |
| Zscore (Magnesium) | 0.778 | −0.699 | 0.456 |
| Zscore (Manganese) | 0.736 | −0.660 | |
| Zscore (Phosphorus) | 0.757 | −0.334 | 0.849 |
| Zscore (Iron) | 0.938 | −0.435 | 0.396 |
| Zscore (Sodium) | 0.590 | 0.514 | |
| Zscore (Potassium) | 0.684 | −0.723 | 0.569 |
| Zscore (Copper) | 0.438 | −0.764 | 0.309 |
| Zscore (Zinc) | 0.865 | 0.522 | |
| Zscore (Retinol) | 0.372 | 0.915 | |
| Zscore (Thiamine) | 0.604 | −0.311 | 0.528 |
| Zscore (Riboflavin) | 0.320 | 0.848 | |
| Zscore (Piridoxine) | |||
| Zscore (Niacin) | 0.465 | 0.362 | |
| Zscore (vitamin C) | −0.811 | ||
| Variables | Cluster 1 “Poor Periodontal Status” | Cluster 2 “Intermediate Periodontal Status” | Cluster 3 “Healthy Periodontal Status” | F Test | p-Value |
|---|---|---|---|---|---|
| Number of cases | 202 | 194 | 54 | ||
| Periodontal disease severity | 1.06 | 0.94 | 0.74 | 3.831 | 0.022 |
| Age | 54.67 | 48.17 | 43.04 | 25.654 | <0.001 |
| Sex | 0.15 | 0.23 | 0.35 | 5.579 | 0.004 |
| BMI | 29.64 | 28.75 | 28.41 | 1.419 | 0.243 |
| Energy intake | 1172.15 | 1706.59 | 2440.00 | 899.059 | <0.001 |
| Micronutrient intake—component 1 | −0.71 | 0.33 | 1.51 | 240.647 | <0.001 |
| Micronutrient intake—component 2 | 0.31 | −0.15 | −0.62 | 22.771 | <0.001 |
| Micronutrient intake—component 3 | −0.44 | 0.17 | 1.08 | 64.526 | <0.001 |
| Physical activity | 0.34 | 0.33 | 0.31 | 0.132 | 0.876 |
| Diabetes mellitus | 0.34 | 0.24 | 0.24 | 2.632 | 0.073 |
| Hypertension | 0.55 | 0.50 | 0.35 | 3.576 | 0.029 |
| Hypercholesterolemia | 0.30 | 0.20 | 0.13 | 5.085 | 0.007 |
| Hypertriglyceridemia | 0.02 | 0.02 | 0.00 | 0.543 | 0.581 |
| Hypothyroidism | 0.07 | 0.07 | 0.02 | 1.007 | 0.366 |
| Liver steatosis | 0.03 | 0.00 | 0.02 | 3.433 | 0.033 |
| Cardiopathy | 0.05 | 0.07 | 0.04 | 0.487 | 0.615 |
| Cancer history | 0.03 | 0.02 | 0.00 | 0.879 | 0.416 |
| Renal disease | 0.00 | 0.01 | 0.00 | 0.137 | 0.872 |
| Vascular disease | 0.00 | 0.01 | 0.00 | 0.659 | 0.518 |
| Depression | 0.08 | 0.09 | 0.02 | 0.502 | 0.606 |
| Smoking | 0.28 | 0.19 | 0.13 | 4.194 | 0.016 |
| Family income | 0.34 | 0.38 | 0.27 | 1.071 | 0.344 |
| Educational level | 0.45 | 0.60 | 0.70 | 7.387 | 0.001 |
| Dental treatment last year | 0.36 | 0.37 | 0.39 | 0.077 | 0.926 |
| Alcohol consumption | 0.07 | 0.11 | 0.20 | 3.829 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.D.; Canaan, J.C.R.; Midori Castelo, P.; Campideli Fonseca, D.; Márcia Pereira-Dourado, S.; Mendonça Murata, R.; Pardi, V.; Pereira, L.J. Influence of Micronutrient Intake, Sociodemographic, and Behavioral Factors on Periodontal Status of Adults Assisted by a Public Health Care System in Brazil: A Cross-Sectional Multivariate Analysis. Nutrients 2021, 13, 973. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030973
Costa PD, Canaan JCR, Midori Castelo P, Campideli Fonseca D, Márcia Pereira-Dourado S, Mendonça Murata R, Pardi V, Pereira LJ. Influence of Micronutrient Intake, Sociodemographic, and Behavioral Factors on Periodontal Status of Adults Assisted by a Public Health Care System in Brazil: A Cross-Sectional Multivariate Analysis. Nutrients. 2021; 13(3):973. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030973
Chicago/Turabian StyleCosta, Patrícia Daniela, Juliana Cristina Reis Canaan, Paula Midori Castelo, Douglas Campideli Fonseca, Stela Márcia Pereira-Dourado, Ramiro Mendonça Murata, Vanessa Pardi, and Luciano José Pereira. 2021. "Influence of Micronutrient Intake, Sociodemographic, and Behavioral Factors on Periodontal Status of Adults Assisted by a Public Health Care System in Brazil: A Cross-Sectional Multivariate Analysis" Nutrients 13, no. 3: 973. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030973