Variation of the Main Alkaloid Content in Equisetum palustre L. in the Light of Its Ontogeny
Abstract
:1. Introduction
- (i)
- Will the factors such as “year” and “site/population” contribute to explain the variance of the alkaloid content?
- (ii)
- Does the ontogeny of E. palustre influence the alkaloid levels?
- (iii)
- Do growing conditions/season play a role in the bioformation of Equisetum-type alkaloids?
2. Results
2.1. Variability of the Equisetum-Type Alkaloid Contents
2.2. Results of the Linear Mixed Model Analysis
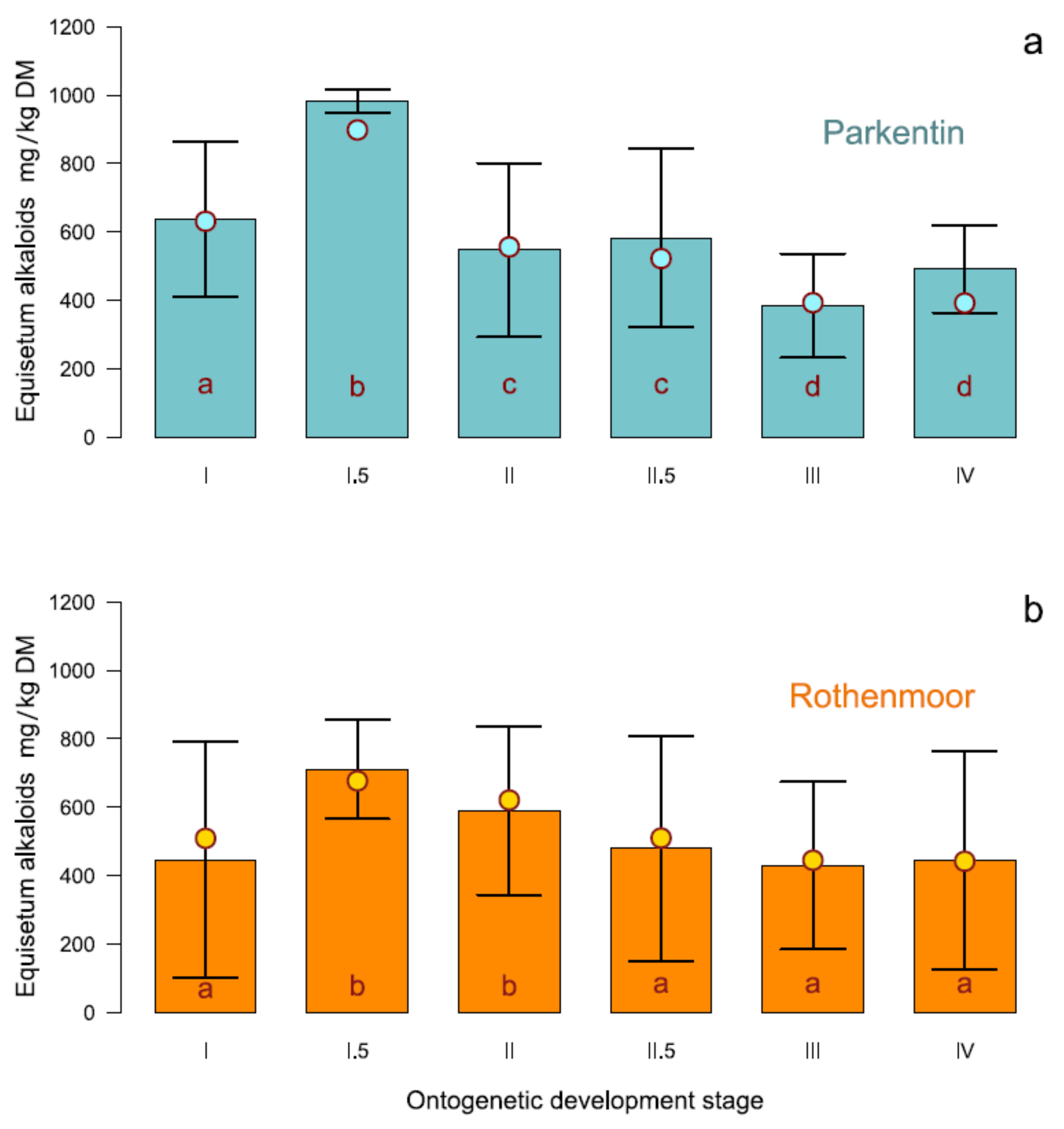
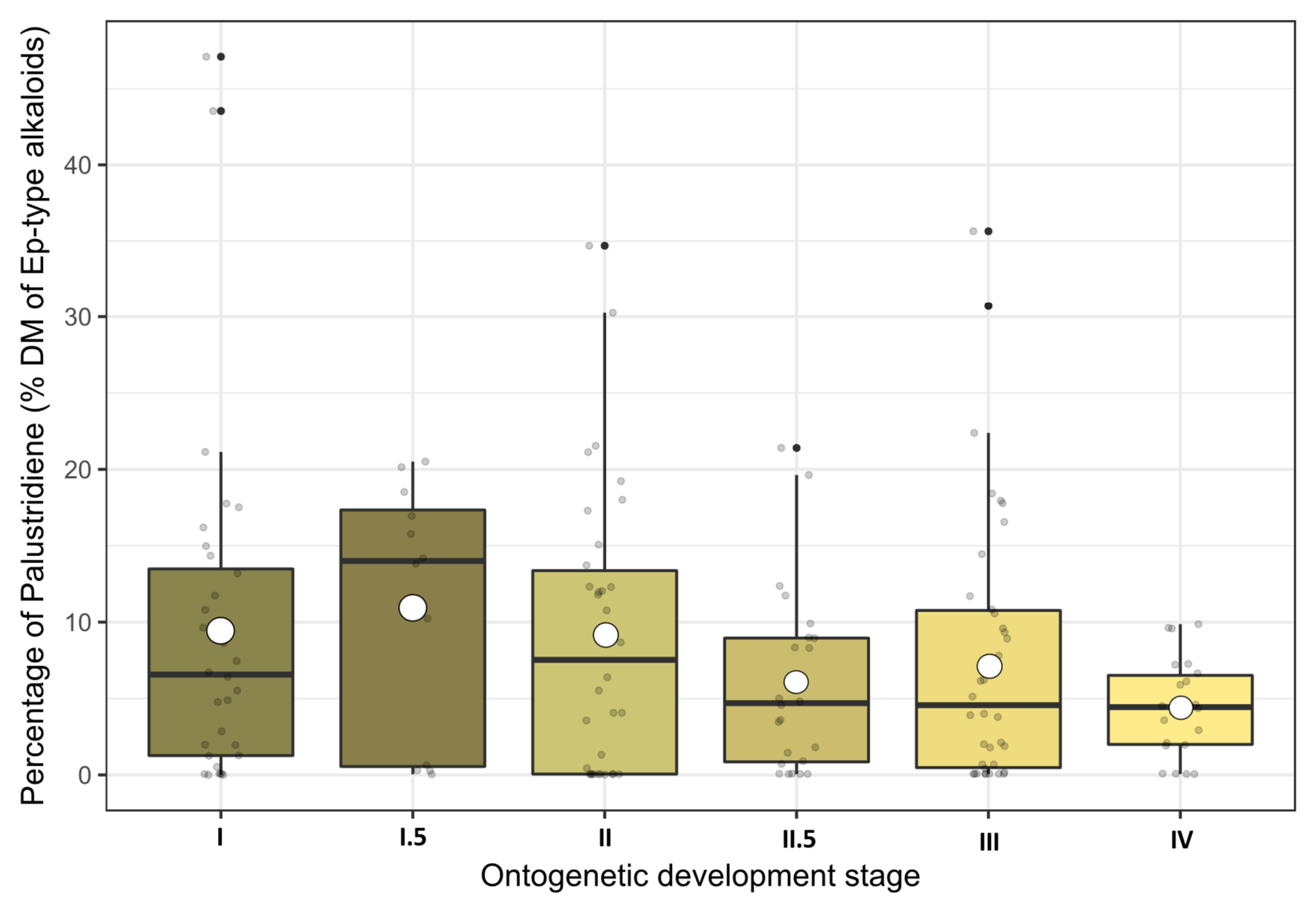
2.3. Effects of the Ontogenetic Stage on Main Alkaloid Contents
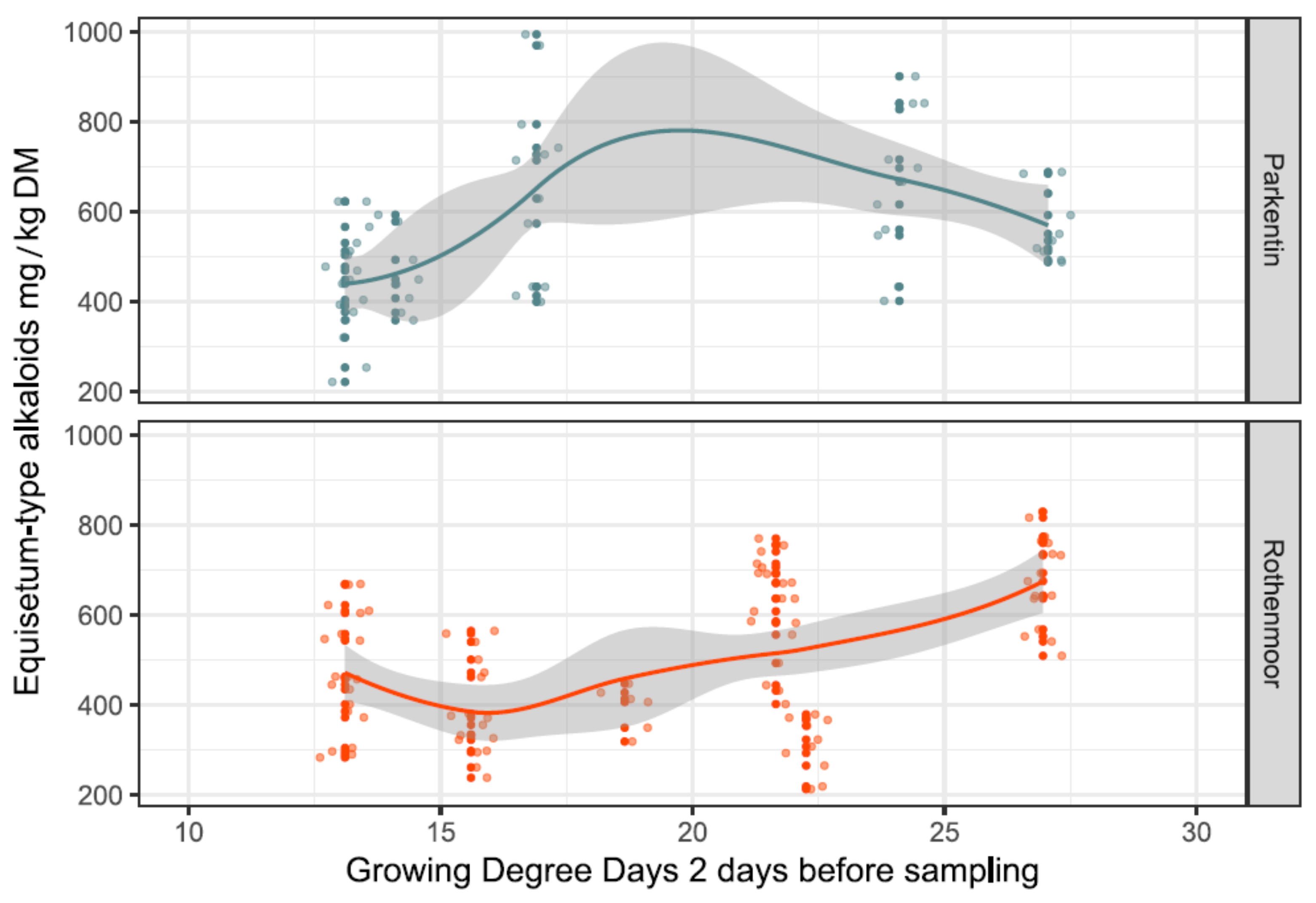
2.4. Effects of Random Variables on Main Alkaloid Contents
3. Discussion
3.1. Variation of Equisetum-Type Alkaloid Levels
3.2. Effect of the Ontogenetic Development on Equisetum-Type Alkaloid Contents
3.3. The Ambiguous Role of Season on Alkaloid Levels and Toxicity
3.4. Aspects of Reducing the Risk of Feed Contamination
4. Conclusions
5. Materials and Methods
5.1. Collection Sites
5.2. Sampling Procedure and Sample Preparations
- (I)
- Early stage, main axis without visible lateral branches;
- (II)
- Lateral leaf branches visible, beginning of whorled phyllotaxis;
- (III)
- Developed whorls, leaf segments still growing;
- (IV)
- Fully developed growth stage, leaf segments are expanded.
5.3. Determination of Covariables
5.4. Alkaloid Analysis
5.4.1. Chemicals and Reagents
5.4.2. Extraction and Sample Preparation
5.4.3. HPLC-ESI-MS/MS Analysis
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hauke, R.L. A Taxonomic Monograph of Equisetum Subgenus Equisetum. Nova Hedwig. 1979, 30, 385–456. [Google Scholar] [CrossRef]
- Knowlton, A. Equisetum. Curr. Biol. 2012, 22, R388–R390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, P.J.V. Ecology of Equisetum palustre in Finland, with special reference to its role as a noxious weed. Ann. Bot. Fenn. 1971, 8, 93–141. [Google Scholar]
- Mukula, J. Studies on the Biology and Control of Marsh Horsetail (Equisetum palustre L.); Maatalouden Tutkimuskeskuksen Aikakauskirja (Annales Agriculturae Fenniae): Helsinki, Finland, 1963; 57p. [Google Scholar]
- Blüml, V.; Lange, G.; Most, A.; Müller, J. Use of wet grassland infested by Equisetum palustre L.: (Nutzungsmöglichkeiten von Feuchtgrünland mit Vorkommen vom Sumpf-Schachtelhalm). Inf. Nat. Niedersachs. 2014, 34, 69–92. [Google Scholar]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Joyce, C.B.; Wade, P.M. (Eds.) European Wet Grasslands. Biodiversity, Management and Restoration; Wiley: Chichester, UK, 1998; ISBN 0471976199. [Google Scholar]
- Weber, C.A. Der Duwock (Equisetum palustre). In Die Bekämpfung des Unkrautes, 2nd ed.; Paul Parey: Berlin, Germany, 1903; 68p. [Google Scholar]
- Forenbacher, S. Schachtelhalmvergiftung der Pferde-eine B1 Avitaminose. Schweiz. Arch. Tierheilkd. 1952, 94, 153–171. [Google Scholar]
- Lindt, S. Horsetail poisoning in calves. Schweiz. Arch. Tierheilkd. 1959, 101, 461–464. [Google Scholar]
- Von Kries, A. Der Sumpfschachtelhalm-Eine Monographie zur Nutzanwendung in der Landwirtschaft; Technische Universität Berlin: Berlin, Germany, 1962; 133p. [Google Scholar]
- Karrer, P.; Eugster, C.H. Über ein Alkaloid aus Equisetum palustre. Helv. Chim. Acta 1948, 31, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S. Über die Alkaloide des Sumpfschachtelhalms (Equisetum palustre). Angew. Chem. 1950, 62, 446–447. [Google Scholar]
- Eugster, C.H.; Dietsche, W.; Baumann, C.G. Partialstruktur für Palustrin (Hauptalkaloid von Equisetum palustre): Partial structure of palustrine (main alkaloid of Equisetum palustre). Angew. Chem. Int. Ed. 1960, 72, 271. [Google Scholar]
- Wohlbier, W.; Beckmann, S. Über die Inhaltsstoffe des Sumpfschachtelhalms (Equisetum palustre). Chem. Ber. 1950, 83, 310–314. [Google Scholar] [CrossRef]
- Hünsche, A.K. Investigations on adverse effects of feeding dried marsh horsetail (Equisetum palustre) Contaminated Forage in Ruminants and Ponies (German with English Summary): Untersuchungen zu möglichen Schadwirkungen einer Kontamination von Grundfutter mit getrocknetem Sumpfschachtelhalm (Equisetum palustre) bei Wiederkäuern und Ponys. Ph.D. Thesis, University of Veterinary Medicine, Hannover, Germany, 2010. [Google Scholar]
- Cramer, L.; Ernst, L.; Lubienski, M.; Papke, U.; Schiebel, H.-M.; Jerz, G.; Beuerle, T. Structural and quantitative analysis of Equisetum alkaloids. Phytochemistry 2015, 116, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Tipke, I.; Bücker, L.; Middelstaedt, J.; Winterhalter, P.; Lubienski, M.; Beuerle, T. HILIC HPLC-ESI-MS/MS identification and quantification of the alkaloids from the genus Equisetum. Phytochem. Anal. 2019, 30, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Holz, W.; Richter, W. The alkaloid content of horsetail (Equisetum palustre L.): (Über den Alkaloidgehalt im Duwock (Equisetum palustre L.)). Angew. Bot. 1960, 34, 28–32. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Lozano, M.C.; Martinez, N.M.; Diaz, G.J. Content of the Saponin Protodioscin in Brachiaria spp. from the Eastern Plains of Colombia. Toxins 2017, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Eller, A.; Chizzola, R. Seasonal variability in pyrrolizidine alkaloids in Senecio inaequidens from the Val Venosta (Northern Italy). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 1306–1312. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, M.; Bowers, M.D. Critical Phenological Events Affect Chemical Defense of Plant Tissues: Iridoid Glycosides in a Woody Shrub. J. Chem. Ecol. 2020, 46, 206–216. [Google Scholar] [CrossRef]
- Uotila, I. On the Injuriousness of Horsetail, and Particularly of Marsh Horsetail (Equisetum palustre L.) in the Feeding of Domestic Animals and on the Distribution of this Species in Finland. (Kortteiden ja Erityisesti Suokortteen (Equisetum palustre L.) Vahingollisuudesta Kotieläinten Ruokinnassa Sekä Tämän Lajin Levinneisyydestä Suomessa). Ann. Agric. Fenn. 1956, 90, 1–155. [Google Scholar]
- Rasran, L.; Jeromin, H. Dominance of particular weed species in relation to nature conservation management on permanent grasslands (review). Telma 2010, 40, 119–136. [Google Scholar]
- Urban, D.; Grzywna, A. Changes of species composition of the Poa pratensis-Festuca rubra plant communities in drainage peatland. J. Water Land Dev. 2017, 35, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Häfner, K.; Zasada, I.; Sagebiel, J. Farmers Preferences for an Agri-Environmental Measure designed for Climate Friendly Peatland Management. In Environmental Economics and Policy; IAAE: Vancouver, BC, Canada, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Aboling, S.; Rottmann, S.; Wolf, P.; Jahn-Falk, D.; Kamphues, J. Case report: Complex plant poisoning in heavily pregnant heifers in Germany. J. Vet. Sci. Technol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [Green Version]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Correia, P.; Bashforth, A.R.; Šimůnek, Z.; Cleal, C.J.; Sá, A.A.; Labandeira, C.C. The History of Herbivory on Sphenophytes: A New Calamitalean with an Insect Gall from the Upper Pennsylvanian of Portugal and a Review of Arthropod Herbivory on an Ancient Lineage. Int. J. Plant Sci. 2020, 181, 387–418. [Google Scholar] [CrossRef]
- Labun, P.; Grulova, D.; Salamon, I.; Šeršeň, F. Calculating the Silicon in Horsetail (Equisetum arvense L.) during the Vegetation Season. FNS 2013, 04, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Kamphues, J. Refusal of breeding bulls to eat hay contaminated with horsetail (Equisetum palustre). Tierarztl. Prax. 1990, 18, 349–351. [Google Scholar]
- Jansen, G.; Jürgens, H.-U.; Ordon, F. Effects of Temperature on the Alkaloid Content of Seeds of Lupinus angustifolius Cultivars. J. Agron. Crop Sci. 2009, 195, 172–177. [Google Scholar] [CrossRef]
- Hennessy, L.M.; Popay, A.J.; Finch, S.C.; Clearwater, M.J.; Cave, V.M. Temperature and Plant Genotype Alter Alkaloid Concentrations in Ryegrass Infected with an Epichloë Endophyte and This Affects an Insect Herbivore. Front. Plant Sci. 2016, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.S. Fundamentals of Radiation and Temperature Relations. In Physiological Plant Ecology I; Springer: Berlin, Germany, 2012; pp. 11–40. [Google Scholar]
- Cop, J.; Vidrih, M.; Hacin, J. Influence of cutting regime and fertilizer application on the botanical composition, yield and nutritive value of herbage of wet grasslands in Central Europe. Grass Forage Sci. 2009, 64, 454–465. [Google Scholar] [CrossRef]
- Guijarro, J.A. Climatol; Climate Tools (Series Homogenization and Derived Products). R-Package Version 3.1.2. 2019. Available online: https://CRAN.R-project.org/package=climatol (accessed on 14 September 2020).
- R Development Core Team. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 24 August 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82. [Google Scholar] [CrossRef] [Green Version]



| Fixed Effect | NDF 1 | DDF 2 | F-Value | p |
|---|---|---|---|---|
| Ontogenetic stage | 5 | 140.82 | 53.51 | <0.001 *** |
| Sampling site | 1 | 149.53 | 1.64 | 0.203 n.s. |
| Interaction stage × site | 5 | 140.91 | 11.17 | <0.001 *** |
| Random Effect | DF 3 | Chi-squared | p | |
| Year of survey | 1 | 0.00 | n.s. | |
| Growing degree days 2 | 1 | 155.97 | <0.001 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, J.; Puttich, P.M.; Beuerle, T. Variation of the Main Alkaloid Content in Equisetum palustre L. in the Light of Its Ontogeny. Toxins 2020, 12, 710. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12110710
Müller J, Puttich PM, Beuerle T. Variation of the Main Alkaloid Content in Equisetum palustre L. in the Light of Its Ontogeny. Toxins. 2020; 12(11):710. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12110710
Chicago/Turabian StyleMüller, Jürgen, Philipp Mario Puttich, and Till Beuerle. 2020. "Variation of the Main Alkaloid Content in Equisetum palustre L. in the Light of Its Ontogeny" Toxins 12, no. 11: 710. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12110710





