Light-Induced Actuation of Poly(dimethylsiloxane) Filled with Graphene Oxide Grafted with Poly(2-(trimethylsilyloxy)ethyl Methacrylate)
Abstract
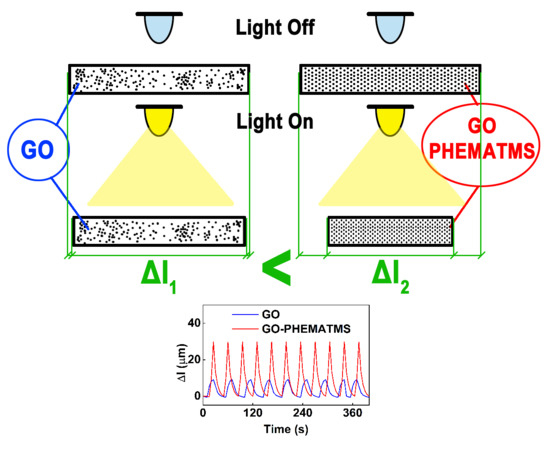
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Initiated Atom Transfer Radical Polymerization
2.3. Elastometric Matrix Preparation
2.4. Analyses
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Uenal, B.; Khademhosseini, A.; Butt, H. Wearables in medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Urbas, A.M.; Li, Q. Soft materials driven by photothermal effect and their applications. Adv. Opt. Mater. 2018, 6, 21. [Google Scholar] [CrossRef]
- He, K.; Wen, Q.K.; Wang, C.W.; Wang, B.X.; Yu, S.S.; Hao, C.C.; Chen, K.Z. A facile synthesis of hierarchical flower-like TiO2 wrapped with MoS2 sheets nanostructure for enhanced electrorheological activity. Chem. Eng. J. 2018, 349, 416–427. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchova, M.; Horsky, J.; Walterova, Z.; Filippov, S.K.; Plachy, T.; Mrlik, M. Oxidation of pyrrole with p-benzoquinone to semiconducting products and their application in electrorheology. New J. Chem. 2018, 42, 10167–10176. [Google Scholar] [CrossRef]
- Hu, T.; Xuan, S.H.; Ding, L.; Gong, X.L. Stretchable and magneto-sensitive strain sensor based on silver nanowire-polyurethane sponge enhanced magnetorheological elastomer. Mater. Des. 2018, 156, 528–537. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Mosnacek, J.; Munster, L.; Pavlinek, V. Synthesis of silicone elastomers containing silyl-based polymer grafted carbonyl iron particles: An efficient way to improve magnetorheological, damping, and sensing performances. Macromolecules 2017, 50, 2189–2200. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Wang, L.F.; Sun, K.; Luo, T.; Yu, Z.Z.; Pan, K. Graphene-based janus film with improved sensitive response capacity for smart actuators. Sens. Actuators B Chem. 2018, 268, 421–429. [Google Scholar] [CrossRef]
- Wang, T.P.; Li, M.T.; Zhang, H.; Sun, Y.Y.; Dong, B. A multi- responsive bidirectional bending actuator based on polypyrrole and agar nanocomposites. J. Mater. Chem. C 2018, 6, 6416–6422. [Google Scholar] [CrossRef]
- Zahoranova, A.; Mrlik, M.; Tomanova, K.; Kronek, J.; Luxenhofer, R. Aba and bab triblock copolymers based on 2-methyl-2-oxazoline and 2-n-propyl-2-oxazoline: Synthesis and thermoresponsive behavior in water. Macromol. Chem. Phys. 2017, 218, 12. [Google Scholar] [CrossRef]
- Shah, A.; Malik, M.S.; Khan, G.S.; Nosheen, E.; Iftikhar, F.J.; Khan, F.A.; Shukla, S.S.; Akhter, M.S.; Kraatz, H.B.; Aminabhavi, T.M. Stimuli-responsive peptide-based biomaterials as drug delivery systems. Chem. Eng. J. 2018, 353, 559–583. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Chen, A.P.; Naidu, A.; Napier, B.S.; Li, W.Y.; Rodriguez, C.L.; Crooker, S.A.; Omenetto, F.G. Flexible magnetic composites for light-controlled actuation and interfaces. Proc. Natl. Acad. Sci. USA 2018, 115, 8119–8124. [Google Scholar] [CrossRef] [PubMed]
- Toshchevikov, V.; Petrova, T.; Saphiannikova, M. Kinetics of ordering and deformation in photosensitive azobenzene lc networks. Polymers 2018, 10, 20. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Slouf, M.; Zhigunov, A.; Koynov, K.; Mosnacek, J. Synthesis of photoactuating acrylic thermoplastic elastomers containing diblock copolymer-grafted carbon nanotubes. ACS Macro Lett. 2014, 3, 999–1003. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, G.; Lan, T.; Zhao, J.J.; Liu, Y.; Chen, W. A graphene-based bimorph structure for design of high performance photoactuators. Adv. Mater. 2015, 27, 7867–7873. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Zhan, W.J.; Peng, R.G.; He, C.G.; Pang, X.C.; Shi, D.; Jiang, T.; Lin, Z.Q. Graphene-enabled superior and tunable photomechanical actuation in liquid crystalline elastomer nanocomposites. Adv. Mater. 2015, 27, 6376–6381. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, F.A.; Fedele, C.; De Martino, S.; Cavalli, S.; Vecchione, R.; Netti, P.A. Three-dimensional microstructured azobenzene-containing gelatin as a photoactuable cell confining system. Acs Appl. Mater. Interfaces 2018, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yun, J.H.; Kim, H.; Cho, M. Light propagation and photoactuation in densely cross-linked azobenzene-functionalized liquid-crystalline polymers: Contribution of host and concerted isomerism. Macromolecules 2016, 49, 6012–6020. [Google Scholar] [CrossRef]
- Osicka, J.; Ilcikova, M.; Mrlik, M.; Minarik, A.; Pavlinek, V.; Mosnacek, J. The impact of polymer grafting from a graphene oxide surface on its compatibility with a pdms matrix and the light-induced actuation of the composites. Polymers 2017, 9, 14. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Doroshenko, M.; Koynov, K.; Danko, M.; Mosnacek, J. Tailoring of viscoelastic properties and light-induced actuation performance of triblock copolymer composites through surface modification of carbon nanotubes. Polymer 2015, 72, 368–377. [Google Scholar] [CrossRef]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-nanoplatelet-based photomechanical actuators. Nanotechnology 2012, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Koerner, H.; Price, G.; Pearce, N.A.; Alexander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites—Stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2004, 3, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Kausar, A.; Siddiq, M. Review highlighting physical prospects of styrenic polymer and styrenic block copolymer reinforced with carbon nanotube. Polym. Plast. Technol. Eng. 2017, 56, 573–593. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Chorvat, D.; Krupa, I.; Slouf, M.; Koynov, K.; Mosnacek, J. Viscoelastic and photo-actuation studies of composites based on polystyrene-grafted carbon nanotubes and styrene-b-isoprene-b-styrene block copolymer. Polymer 2014, 55, 211–218. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Qin, M.M.; Guo, H.Q.; Yoshino, K.; Feng, W. Infrared-actuated recovery of polyurethane filled by reduced graphene oxide/carbon nanotube hybrids with high energy density. Acs Appl. Mater. Interfaces 2013, 5, 10882–10888. [Google Scholar] [CrossRef] [PubMed]
- Czanikova, K.; Torras, N.; Esteve, J.; Krupa, I.; Kasak, P.; Pavlova, E.; Racko, D.; Chodak, I.; Omastova, M. Nanocomposite photoactuators based on an ethylene vinyl acetate copolymer filled with carbon nanotubes. Sens. Actuators B 2013, 186, 701–710. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Sedlacik, M.; Mosnacek, J.; Peer, P.; Filip, P. Cholesteryl-coated carbonyl iron particles with improved anti-corrosion stability and their viscoelastic behaviour under magnetic field. Colloid Polym. Sci. 2014, 292, 2137–2143. [Google Scholar] [CrossRef]
- Osicka, J.; Mrlik, M.; Ilcikova, M.; Hanulikova, B.; Urbanek, P.; Sedlacik, M.; Mosnacek, J. Reversible actuation ability upon light stimulation of the smart systems with controllably grafted graphene oxide with poly (glycidyl methacrylate) and pdms elastomer: Effect of compatibility and graphene oxide reduction on the photo-actuation performance. Polymers 2018, 10, 832. [Google Scholar] [CrossRef]
- Mrlik, M.; Cvek, M.; Osicka, J.; Moucka, R.; Sedlacik, M.; Pavlinek, V. Surface-initiated atom transfer radical polymerization from graphene oxide: A way towards fine tuning of electric conductivity and electro-responsive capabilities. Mater. Lett. 2018, 211, 138–141. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Moucka, R.; Pavlinek, V.; Mosnacek, J. Tunable electrorheological performance of silicone oil suspensions based on controllably reduced graphene oxide by surface initiated atom transfer radical polymerization of poly(glycidyl methacrylate). J. Ind. Eng. Chem. 2018, 57, 104–112. [Google Scholar] [CrossRef]
- Yoon, J.T.; Lee, S.C.; Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos. Sci. Technol. 2010, 70, 776–782. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Spitalsky, Z.; Micusik, M.; Csomorova, K.; Sasinkova, V.; Kleinova, A.; Mosnacek, J. A tertiary amine in two competitive processes: Reduction of graphene oxide vs. Catalysis of atom transfer radical polymerization. Rsc Adv. 2015, 5, 3370–3376. [Google Scholar] [CrossRef]
- Ma, C.X.; Le, X.X.; Tang, X.L.; He, J.; Xiao, P.; Zheng, J.; Xiao, H.; Lu, W.; Zhang, J.W.; Huang, Y.J.; et al. A multiresponsive anisotropic hydrogel with macroscopic 3d complex deformations. Adv. Funct. Mater. 2016, 26, 8670–8676. [Google Scholar] [CrossRef]









| Sample Name | Ea α′ (kJ·mol−1) | Ea α (kJ·mol−1) |
|---|---|---|
| PDMS | 19.96 | 45.70 |
| PDMS/GO 0.1% | 19.04 | 36.57 |
| PDMS/GO-PHEMATMS 0.1% | 10.7 | 22.01 |
| PDMS/GO-PHEMATMS 0.5% | 9.23 | 21.3 |
| PDMS/GO-PHEMATMS 1% | 9.09 | 20.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osicka, J.; Mrlik, M.; Ilčíková, M.; Munster, L.; Bazant, P.; Špitalský, Z.; Mosnáček, J. Light-Induced Actuation of Poly(dimethylsiloxane) Filled with Graphene Oxide Grafted with Poly(2-(trimethylsilyloxy)ethyl Methacrylate). Polymers 2018, 10, 1059. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101059
Osicka J, Mrlik M, Ilčíková M, Munster L, Bazant P, Špitalský Z, Mosnáček J. Light-Induced Actuation of Poly(dimethylsiloxane) Filled with Graphene Oxide Grafted with Poly(2-(trimethylsilyloxy)ethyl Methacrylate). Polymers. 2018; 10(10):1059. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101059
Chicago/Turabian StyleOsicka, Josef, Miroslav Mrlik, Markéta Ilčíková, Lukas Munster, Pavel Bazant, Zdenko Špitalský, and Jaroslav Mosnáček. 2018. "Light-Induced Actuation of Poly(dimethylsiloxane) Filled with Graphene Oxide Grafted with Poly(2-(trimethylsilyloxy)ethyl Methacrylate)" Polymers 10, no. 10: 1059. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101059