Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
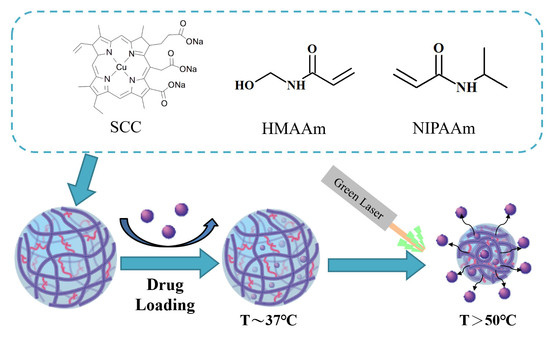
2.2. Synthesis of p(NIPAAm-co-NHMAAm-co-SCC) Nanogels
2.3. Characterization of Nanogels
2.4. Cytotoxicity of Nanogels
2.5. Drug Loading and Release
2.6. Green Laser-Induced Cell Death in the Presence of the Nanogels
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Poly(NIPAAm-co-NHMAAM-co-SCC) Nanogels
3.2. Photothermal Property of the Nanogels
3.3. Green Laser-Induced Cell Death via the Photo-Thermal Effect of the Nanogels
3.4. Drug Loading to and Release from the Nanogel
3.5. The Cell-Killing Efficacy of 5-FU-Loaded Nanogels under Green Laser Irradiation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. Ca-Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Sign. Transdu. Targ. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar] [PubMed]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215. [Google Scholar] [CrossRef]
- Raju, R.; Bandyopadhyay, S.; Sharma, A.; Gonzalez, S.V.; Carlsen, P.H.; Gautun, O.R.; Glomm, W.R. Synthesis, characterization and drug loading of multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Baek, S.; Singh, R.K.; Kim, T.H.; Seo, J.W.; Shin, U.S.; Chrzanowski, W.; Kim, H.W. Triple hit with drug carriers: pH- and temperature-responsive theranostics for multimodal chemo- and photothermal therapy and diagnostic applications. ACS Appl. Mater. Interfaces 2016, 8, 8967–8979. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.E.; Dahotre, S.N.; West, J.L. Hydrogel-nanoparticle composites for optically modulated cancer therapeutic delivery. J. Contr. Rel. 2014, 178, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Delivery Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.B.; Liu, J.J.; Wu, Y.F.; Feng, L.Z.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320, 100–117. [Google Scholar] [CrossRef]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Hajiesmaeilbaigi, F.; Razzaghi, H.; Mahdizadeh, M.; Moghaddam, M.R.A.; Ruzbehani, M. Design and construction of a 110 W green laser for medical application. Opt. & Las. Technol. y 2011, 43, 1428–1430. [Google Scholar] [CrossRef]
- Müller, G.J.; Berlien, P.; Scholz, C. The medical laser. Med. Las. Appl. 2006, 21, 99–108. [Google Scholar] [CrossRef]
- Pegau, W.S.; Gray, D.; Zaneveld, J.R.V. Absorption and attenuation of visible and near-infrared light in water: Dependence on temperature and salinity. Appl. Opt. 1997, 36, 6035–6046. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Hsu, C.F.; Tsai, W.B. Fabrication of chlorophyll-incorporated nanogels for potential applications in photothermal cancer therapy. ACS Omega 2018. under revision. [Google Scholar]
- Wang, X.; Li, S.; Wan, Z.; Quan, Z.; Tan, Q. Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int. J. Pharm. 2014, 463, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bazin, G.; Zhu, X.X. Understanding the thermo-sensitivity of crystalline colloidal arrays formed by poly(styrene-co-N-isopropylacrylamide) core-shell microspheres. Soft Mat. 2012, 8, 1909–1915. [Google Scholar] [CrossRef]
- Chien, H.-W.; Tsai, W.-B.; Jiang, S. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials 2012, 33, 5706–5712. [Google Scholar] [CrossRef] [PubMed]
- Dowding, P.J.; Vincent, B.; Williams, E. Preparation and swelling properties of poly(NIPAM) “minigel” particles prepared by inverse suspension polymerization. J. Colloid Interface Sci. 2000, 221, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Chiu, W.-Y.; Lee, C.-F. Preparation, morphology, and thermoresponsive properties of poly(N-isopropylacrylamide)-based copolymer microgels. J. Polym. Sci. Part A 2006, 44, 356–370. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Q.C.; Zhao, H.; Zhang, L.; Li, J.F.; Gao, J.; Qian, W.Z.; Li, B.H.; Chen, H.W.; Wang, H.; et al. Novel dual-control poly(N-isopropylacrylamide-co-chlorophyllin) nanogels for improving drug release. Nanomedicine 2012, 7, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ye, Y.W.; Zhu, A.L.; Yang, Z.; Fu, Y.; Wei, C.Q.; Liu, Q.; Zhao, C.L.; Wang, G.J.; Zhang, X.F. Hyperthermia combined with 5-fluorouracil promoted apoptosis and enhanced thermotolerance in human gastric cancer cell line sgc-7901. OncoTargets Ther. 2015, 8, 1265–1270. [Google Scholar] [CrossRef]
- Cheewatanakornkool, K.; Niratisai, S.; Manchun, S.; Dass, C.R.; Sriamornsak, P. Characterization and in vitro release studies of oral microbeads containing thiolated pectin–doxorubicin conjugates for colorectal cancer treatment. Asian J. Pharm. Sci. 2017, 12, 509–520. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T.H.; Bohling, A.; Vinogradov, S.V. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: Synthesis, pharmacokinetics, and tumor growth inhibition. Mol. Pharm. 2014, 11, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Lee, T.Y.; Fu, P.C.; Lo, C.L.; Chiang, Y.T. Multifunctional polymer nanoparticles for dual drug release and cancer cell targeting. Polymers 2017, 9, 213. [Google Scholar] [CrossRef]
- Vossen, L.I.; Wedepohl, S.; Calderon, M. A facile, one-pot, surfactant-free nanoprecipitation method for the preparation of nanogels from polyglycerol-drug conjugates that can be freely assembled for combination therapy applications. Polymers 2018, 10, 398. [Google Scholar] [CrossRef]
- Su, S.; Wang, H.; Liu, X.; Wu, Y.; Nie, G. Irgd-coupled responsive fluorescent nanogel for targeted drug delivery. Biomaterials 2013, 34, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-B.; Lai, H.-Y.; Lee, J.-L.; Lo, C.-W.; Chen, W.-S. Enhancement of the cytotoxicity and selectivity of doxorubicin to hepatoma cells by synergistic combination of galactose-decorated γ-poly(glutamic acid) nanoparticles and low-intensity ultrasound. Langmuir 2014, 30, 5510–5517. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, R.; Tsai, W.-B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101098
Chang R, Tsai W-B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers. 2018; 10(10):1098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101098
Chicago/Turabian StyleChang, Ray, and Wei-Bor Tsai. 2018. "Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy" Polymers 10, no. 10: 1098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101098