Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Experimental and HA Resin Adhesives
2.2. Characterization of HA and Synthesized Adhesive
2.3. Tooth Preparation and Bonding Procedure
2.4. Micro-Tensile Bond Strength (μTBS)
2.5. Micro-Raman Spectroscopy of Dentin–Adhesive Bonded Specimen
2.6. SEM Observation of Bonded Adhesive/Dentin Interface
2.7. Degree of Conversion (DC)
- Caliphatic is 1638 cm−1 absorption peak of polymerized resin,
- Caromatic is 1607 cm−1 absorption peak of polymerized resin,
- Ualiphatic is 1638 cm−1 absorption peak of unpolymerized resin and
- Uaromatic is 1607 cm−1 absorption peak of unpolymerized resin.
3. Results
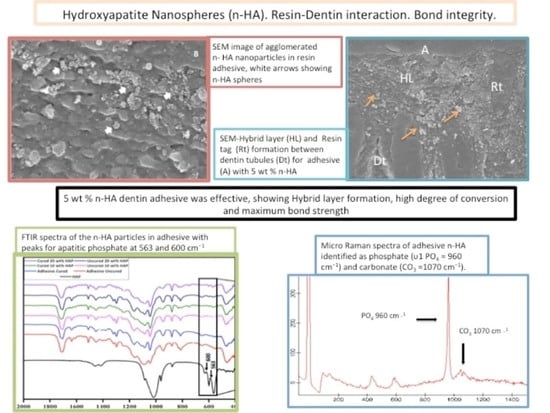
3.1. SEM/EDX Outcomes
3.2. Microtensile Bond Strength (μTBS)
3.3. FTIR Analysis
3.4. Micro-Raman Spectroscopy Outcomes
3.5. Degree of Conversion (DC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farooq, I.; Moheet, I.A.; AlShwaimi, E. In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch. Oral Biol. 2015, 60, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Farooq, I.; Alakrawi, K.M.; Khalid, H.; Saadi, O.W.; Hakeem, A.S. Dentin tubule occlusion potential of novel dentifrices having fluoride containing bioactive glass and zinc oxide nanoparticles. Med. Princ. Pract. 2020, 29, 338–346. [Google Scholar] [CrossRef]
- Rikame, V.; Doshi, Y.; Horowitz, R.A.; Kevadia-Shah, V.; Shah, M. Comparative evaluation of fluoridated mouthwash and sodium bicarbonate in management of dentin hypersensitivity: An in vitro SEM study. Compend. Contin. Educ. Dent. 2018, 39, e5–e8. [Google Scholar] [PubMed]
- Warreth, A.; Abuhijleh, E.; Almaghribi, M.A.; Mahwal, G.; Ashawish, A. Tooth surface loss: A review of literature. Saudi Dent. J. 2020, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Models of caries formation around dental composite restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.H.; Zhang, L.; Yu, F.; Li, F.; Liu, Z.-Y.; Chen, J.-H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the bonding stability of an etch-and-rinse adhesive to dentin. Materials 2017, 10, 183. [Google Scholar] [CrossRef]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion–not there yet. Jpn. Dent. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Kumari, R.V.; Siddaraju, K.; Nagraj, H.; Poluri, R.K. Evaluation of shear bond strength of newer bonding systems on superficial and deep dentin. J. Int. Oral. Health 2015, 7, 31–35. [Google Scholar]
- Zhang, Z.; Gupte, M.J.; Ma, P.X. Biomaterials and stem cells for tissue engineering. Expert Opin. Biol. Ther. 2013, 13, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar] [PubMed]
- Marrelli, M.; Pujia, A.; Palmieri, F.; Gatto, R.; Falisi, G.; Gargari, M.; Caruso, S.; Apicella, D.; Rastelli, C.; Nardi, G.M. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with CAD-CAM technology. Int. J. Immunopathol. Pharmacol. 2016, 29, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Niu, L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Makvandi, P.; Corcione, C.E.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2018, 29, 364–371. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L.N. Advances in antimicrobial organic and inorganic nanocompounds in biomedicine. Adv. Ther. 2020, 3, 2000024. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Hashemi, P.M.; Imani, R. Characterization of a novel nanobiomaterial fabricated from HA, TiO2 and Al2O3 powders: An in vitro study. Prog. Biomater. 2014, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Liu, Y.; Tjaderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Leitune, V.C.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M.W. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [PubMed]
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H. Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral. Sci. 2019, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaker, R.P.; Ren, G. Potential impact of nanotechnology on the control of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Skrtic, D.; Antonucci, J.M. Polymeric dental composites based on remineralizing amorphous calcium phosphate fillers. Curr. Trends Polym. Sci. 2016, 17, 1–31. [Google Scholar]
- Sadat-Shojai, A.M.; Nodehi, A.; Khanlar, L.N. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482. [Google Scholar] [CrossRef]
- Kavrik, F.; Kucukyilmaz, E. The effect of different ratios of nano-sized hydroxyapatite fillers on the micro-tensile bond strength of an adhesive resin. Microsc. Res. Tech. 2019, 82, 538–543. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P.; Wang, Y.; Misra, A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. A 2007, 80, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral. Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Jose, P.; Sakhamuri, S.; Sampath, V.; Kavitha, S.; Mahalaxmi, S. Degree of conversion of two dentin bonding agents with and without a desensitizing agent using fourier transform infrared spectroscopy: An in vitro study. J. Conserv. Dent. 2011, 14, 302–305. [Google Scholar]
- Can-Karabulut, D.C.; Oz, F.T.; Karabulut, B.; Batmaz, I.; Ilk, O. Adhesion to primary and permanent dentin and a simple model approach. Eur. J. Dent. 2009, 3, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeGeros, R.Z.; Trautz, O.R.; Klein, E.; LeGeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Sepulveda, P.; Hench, L.L. Dose-dependent behavior of bioactive glass dissolution. J. Biomed. Mater. Res. 2001, 58, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Solhi, L.; Atai, M.; Nodehi, A.; Mohammad, I. A novel dentin bonding system containing poly(methacrylic acid) grafted nanoclay: Synthesis, characterization and properties. Dent. Mater. 2012, 28, 1041–1050. [Google Scholar] [CrossRef]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomater 2020, 10, 1466. [Google Scholar] [CrossRef]
- Felemban, N.H.; Ebrahim, M.I. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral. Health 2017, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.-S.; Roh, J.; Sauro, S.; Kim, H.-W.; Lee, J.-H. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef] [Green Version]
- Nobre, C.M.G.; Putz, N.; Hannig, M. Adhesion of hydroxyapatite nanoparticles to dental materials under oral conditions. Scanning 2020, 2020, 6065739. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Sano, H.; Chowdhury, A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef]
- Sirisha, K.; Rambabu, T.; Ravishankar, Y.R.; Ravikumar, P. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.A.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.H.K.; Sun, L.; Weir, M.D.; Antonucci, J.M.; Takagi, S.; Chow, L.C.; Peltz, M. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J. Dent. Res. 2006, 85, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.; Belli, R.; Stotzel, C.; Hilpert, A.; Müller, F.A.; Lohbauer, U. Biomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J. Adhes. Dent. 2013, 15, 413–422. [Google Scholar] [PubMed]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral. Rehab. 2004, 31, 911–917. [Google Scholar] [CrossRef]
- Mortazavi, V.; Fathi, M.; Ataei, E.; Khodaeian, N.; Askari, N. Shear bond strengths and morphological evaluation of filled and unfilled adhesive interfaces to enamel and dentine. Int. J. Dent. 2012, 2012, 858459. [Google Scholar] [CrossRef] [Green Version]
- Anchieta, R.B.; Oliveira, F.G.; Sundfeld, R.H.; Rahal, V.; Rocha, E.P. Analysis of hybrid layer thickness, resin tag length and their correlation with microtensile bond strength using a total etch adhesive to intact dentin. Acta Odontol. Latinoam. 2011, 24, 272–278. [Google Scholar]
- Rangan, S.; Schulze, H.G.; Vardaki, M.Z.; Blades, M.W.; Piret, J.M.; Turner, R.F.B. Applications of Raman spectroscopy in the development of cell therapies: State of the art and future perspectives. Analyst 2020, 145, 2070–2105. [Google Scholar] [CrossRef]
- Miyazaki, M.; Onose, H.; Iida, N.; Kazama, H. Determination of residual double bonds in resin-dentin interface by Raman spectroscopy. Dent. Mater. 2003, 19, 245–251. [Google Scholar] [CrossRef]
- Van-Meerbeek, B.; Mohrbacher, H.; Celis, J.P.; Roos, J.R.; Vanherle, G. Chemical characterization of the resin-dentin interface by micro-Raman spectroscopy. J. Dent. Res. 1993, 72, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y. The effect of hydroxyapatite presence on the degree of conversion and polymerization rate in a model self-etching adhesive. Dent. Mater. 2012, 28, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utneja, S.; Talwar, S.; Nawal, R.R.; Sapra, S.; Mittal, M.; Rajain, A.; Verma, M. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J. Conserv. Dent. 2018, 21, 681–690. [Google Scholar] [CrossRef] [PubMed]






| μTBS (MPa) (Mean ± SD) | Failure Mode Analysis (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | NTC | TC | p-Value * | Adhesive | Cohesive | Mixed |
| CEA | 27.36 ± 2.80 a A | - | <0.01 | 80 | 0 | 20 |
| - | 21.71 ± 3.85 a B | 100 | 0 | 0 | ||
| HA (5%) | 32.50 ± 3.47 bA | 60 | 20 | 20 | ||
| - | 25.31 ± 3.84 a B | 80 | 0 | 20 | ||
| HA (10%) | 24.41 ± 3.72 a A | 70 | 0 | 30 | ||
| - | 17.60 ± 4.36 b B | 100 | 0 | 0 | ||
| Group | Degree of Conversion (Mean ± SD) | Tukey (p < 0.05) |
|---|---|---|
| CEA | 62.3 ± 5.2 | A |
| HA (5 %) | 58.1 ± 4.8 | A |
| HA (10 %) | 51.8 ± 5.6 | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122948
Al-Hamdan RS, Almutairi B, Kattan HF, Alsuwailem NA, Farooq I, Vohra F, Abduljabbar T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers. 2020; 12(12):2948. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122948
Chicago/Turabian StyleAl-Hamdan, Rana S, Basil Almutairi, Hiba F Kattan, Noura A. Alsuwailem, Imran Farooq, Fahim Vohra, and Tariq Abduljabbar. 2020. "Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study" Polymers 12, no. 12: 2948. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122948