Fabrication of Nanoscale Oxide Textured Surfaces on Polymers
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Substrate Preparation
2.3. BCP Templates
2.4. Sequential Infiltration Synthesis
2.5. Passivation Process
2.6. Reactive Ion Etching
2.7. Characterizations
3. Results and Discussion
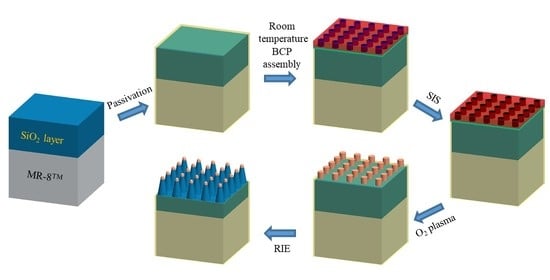
3.1. Process Overview
3.2. Room-Temperature BCP Assembly
3.2.1. Random Copolymer Mat Crosslinking Using UV Treatment
3.2.2. BCP Solvent Annealing
3.2.3. SIS on BCP/SiO2/MR-8 Polymer
3.2.4. Pattern Transfer into SiO2 Underlying Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghenadii, K.; Daniela, N.; Ana, P.; Lidia, S.; Pedro, B.; Luis, P.; Elvira, F.; Rodrigo, M. Metal Oxide Nanostructures Synthesis; Properties and Applications Available online; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Man Ching Ng, A. ZnO Nanostructures: Growth, Properties and Applications. J. Mater. Chem. 2012, 22, 6526. [Google Scholar] [CrossRef]
- Clavijo, W.P.; Atkinson, G.M.; Castano, C.E.; Pestov, D. Novel Low-Temperature Fabrication Process for Integrated High-Aspect Ratio Zinc Oxide Nanowire Sensors. J. Vac. Sci. Technol. B 2016, 34, 022203. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Hahn, Y.-B. Solution Process Synthesis of High Aspect Ratio ZnO Nanorods on Electrode Surface for Sensitive Electrochemical Detection of Uric Acid. Sci. Rep. 2017, 7, 46475. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.A.; Birenbaum, N.S.; Meyer, G.J. Biological Applications of High Aspect Ratio Nanoparticles. J. Mater. Chem. 2004, 14, 517–526. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and Bioinspired Nanostructured Bactericidal Surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Ghanashyam Krishna, M.; Vinjanampati, M.; Dhar Purkayastha, D. Metal Oxide Thin Films and Nanostructures for Self-Cleaning Applications: Current Status and Future Prospects. Eur. Phys. J. Appl. Phys. 2013, 62, 30001. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Qi, L. Recent Advances in Anti-reflective Surfaces Based on Nanostructure Arrays. Mater. Horiz. 2014, 2, 37–53. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, X.-D.; Huang, J.; Sun, L.-X.; Geng, F.; Yi, Z.; Zu, X.-T.; Wu, W.-D.; Zheng, W. Subwavelength Structures for High Power Laser Antireflection Application on Fused Silica by One-Step Reactive Ion Etching. Opt. Lasers Eng. 2016, 78, 48–54. [Google Scholar] [CrossRef]
- Bacon-Brown, D.A.; Braun, P.V. Tunable Antireflection Coating to Remove Index-Matching Requirement for Interference Lithography. Adv. Opt. Mater. 2018, 6, 1701049. [Google Scholar] [CrossRef]
- Kumar Raut, H.; Anand Ganesh, V.; Sreekumaran Nair, A.; Ramakrishna, S. Anti-Reflective Coatings: A Critical, in-Depth Review. Energy Environ. Sci. 2011, 4, 3779–3804. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Hsu, J.-J.; Nien, C.-K.; Yu, H.H. Moth-Eye-Inspired Biophotonic Surfaces with Anti-reflective and Hydrophobic Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 32021–32030. [Google Scholar] [CrossRef]
- Lee, K.; Kreider, M.; Bai, W.; Cheng, L.-C.; Dinachali, S.S.; Tu, K.-H.; Huang, T.; Ntetsikas, K.; Liontos, G.; Avgeropoulos, A.; et al. UV-Solvent Annealing of PDMS-Majority and PS-Majority PS-b-PDMS Block Copolymer Films. Nanotechnology 2016, 27, 465301. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Y.; Wang, Z.; Fan, Y.; Zhang, X. Investigation into an Alternating Multilayer Film of Poly(4-Vinylpyridine) and Poly(Acrylic Acid) Based on Hydrogen Bonding. Langmuir 1999, 15, 1360–1363. [Google Scholar] [CrossRef]
- Jin, B.; He, J.; Yao, L.; Zhang, Y.; Li, J. Rational Design and Construction of Well-Organized Macro-Mesoporous SiO2/TiO2 Nanostructure toward Robust High-Performance Self-Cleaning Antireflective Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 17466–17475. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Park, J.; Lim, H. Improved Antireflection Properties of Moth Eye Mimicking Nanopillars on Transparent Glass: Flat Antireflection and Color Tuning. Nanoscale 2012, 4, 4603–4610. [Google Scholar] [CrossRef]
- Phillips, B.M.; Jiang, P. Chapter 12—Biomimetic Antireflection Surfaces. In Engineered Biomimicry; Lakhtakia, A., Martín-Palma, R.J., Eds.; Elsevier: Boston, MA, USA, 2013; pp. 305–331. [Google Scholar]
- Li, J.; Zhu, J.; Gao, X. Bio-Inspired High-Performance Antireflection and Antifogging Polymer Films. Small 2014, 10, 2578–2582. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Paul, B.K.; Chang, C. Nanostructured ZnO as Biomimetic Anti-Reflective Coatings on Textured Silicon Using a Continuous Solution Process. J. Mater. Chem. 2012, 22, 22906–22912. [Google Scholar] [CrossRef]
- Raut, H.K.; Dinachali, S.S.; He, A.Y.; Ganesh, V.A.; Saifullah, M.S.M.; Law, J.; Ramakrishna, S. Robust and Durable Polyhedral Oligomeric Silsesquioxane-Based Antireflective Nanostructures with Broadband Quasi-Omnidirectional Properties. Energy Environ. Sci. 2013, 6, 1929–1937. [Google Scholar] [CrossRef]
- Baquedano, E.; Torné, L.; Caño, P.; Postigo, P.A. Increased Efficiency of Solar Cells Protected by Hydrophobic and Hydrophilic Anti-Reflecting Nanostructured Glasses. Nanomaterials 2017, 7, 437. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Zhang, G.; Lin, F.; Zhang, J.; Liu, Z.; Mu, S. The Fabrication of Subwavelength Anti-Reflective Nanostructures Using a Bio-Template. Nanotechnology 2008, 19, 095605. [Google Scholar] [CrossRef]
- Rahman, A.; Ashraf, A.; Xin, H.; Tong, X.; Sutter, P.; Eisaman, M.D.; Black, C.T. Sub-50-Nm Self-Assembled Nanotextures for Enhanced Broadband Antireflection in Silicon Solar Cells. Nat. Commun. 2015, 6, 5963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingslake, R.; Barry Johnson, R. Chapter 1—The Work of the Lens Designer. In Lens Design Fundamentals (Second Edition); Kingslake, R., Barry Johnson, R., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 1–23. [Google Scholar]
- Peng, Q.; Tseng, Y.-C.; Darling, S.B.; Elam, J.W. A Route to Nanoscopic Materials via Sequential Infiltration Synthesis on Block Copolymer Templates. ACS Nano 2011, 5, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.Z.; Losego, M.D. Vapor Phase Infiltration (VPI) for Transforming Polymers into Organic–Inorganic Hybrid Materials: A Critical Review of Current Progress and Future Challenges. Mater. Horiz. 2017, 4, 747–771. [Google Scholar] [CrossRef]
- Brassat, K.; Lindner, J.K.N. Nanoscale Block Copolymer Self-Assembly and Microscale Polymer Film Dewetting: Progress in Understanding the Role of Interfacial Energies in the Formation of Hierarchical Nanostructures. Adv. Mater. Interfaces 2020, 7, 1901565. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Darling, S.B. Block Copolymer Nanostructures for Technology. Polymers 2010, 2, 470–489. [Google Scholar] [CrossRef] [Green Version]
- Reid, B.; Taylor, A.; Chen, Y.; Schmidt-Hansberg, B.; Guldin, S. Robust Operation of Mesoporous Antireflective Coatings under Variable Ambient Conditions. ACS Appl. Mater. Interfaces 2018, 10, 10315–10321. [Google Scholar] [CrossRef] [Green Version]
- Päivänranta, B.; Sahoo, P.K.; Tocce, E.; Auzelyte, V.; Ekinci, Y.; Solak, H.H.; Liu, C.-C.; Stuen, K.O.; Nealey, P.F.; David, C. Nanofabrication of Broad-Band Antireflective Surfaces Using Self-Assembly of Block Copolymers. ACS Nano 2011, 5, 1860–1864. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Peng, Q.; Ocola, L.E.; Elam, J.W.; Darling, S.B. Enhanced Block Copolymer Lithography Using Sequential Infiltration Synthesis. J. Phys. Chem. C 2011, 115, 17725–17729. [Google Scholar] [CrossRef]
- Peng, Q.; Tseng, Y.-C.; Darling, S.B.; Elam, J.W. Nanoscopic Patterned Materials with Tunable Dimensions via Atomic Layer Deposition on Block Copolymers. Adv. Mater. 2010, 22, 5129–5133. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. New Insight into the Mechanism of Sequential Infiltration Synthesis from Infrared Spectroscopy. Chem. Mater. 2014, 26, 6135–6141. [Google Scholar] [CrossRef]
- Choi, J.W.; Li, Z.; Black, C.T.; Sweat, D.P.; Wang, X.; Gopalan, P. Patterning at the 10 Nanometer Length Scale Using a Strongly Segregating Block Copolymer Thin Film and Vapor Phase Infiltration of Inorganic Precursors. Nanoscale 2016, 8, 11595–11601. [Google Scholar] [CrossRef] [PubMed]
- Weisbord, I.; Shomrat, N.; Azoulay, R.; Kaushansky, A.; Segal-Peretz, T. Understanding and Controlling Polymer–Organometallic Precursor Interactions in Sequential Infiltration Synthesis. Chem. Mater. 2020, 32, 4499–4508. [Google Scholar] [CrossRef]
- Leng, C.Z.; Losego, M.D. A Physiochemical Processing Kinetics Model for the Vapor Phase Infiltration of Polymers: Measuring the Energetics of Precursor-Polymer Sorption, Diffusion, and Reaction. Phys. Chem. Chem. Phys. 2018, 20, 21506–21514. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.-Y.; Stein, A.; Kisslinger, K.; Black, C.T. Electrical and Structural Properties of ZnO Synthesized via Infiltration of Lithographically Defined Polymer Templates. Appl. Phys. Lett. 2015, 107, 203106. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Xu, Q.; Wang, Z.; Yao, X.; Wang, Y. Highly Ordered TiO2 Nanostructures by Sequential Vapour Infiltration of Block Copolymer Micellar Films in an Atomic Layer Deposition Reactor. J. Mater. Chem. C 2013, 1, 1029–1036. [Google Scholar] [CrossRef]
- Barick, B.K.; Simon, A.; Weisbord, I.; Shomrat, N.; Segal-Peretz, T. Tin Oxide Nanostructure Fabrication via Sequential Infiltration Synthesis in Block Copolymer Thin Films. J. Colloid Interface Sci. 2019, 557, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gunkel, I.; Russell, T.P. Pattern Transfer Using Block Copolymers. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2013, 371, 20120306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Segal-Peretz, T.; Oruc, M.E.; Suh, H.S.; Wu, G.; Nealey, P.F. Fabrication of Nanoporous Alumina Ultrafiltration Membrane with Tunable Pore Size Using Block Copolymer Templates. Adv. Funct. Mater. 2017, 27, 1701756. [Google Scholar] [CrossRef]
- Berman, D.; Guha, S.; Lee, B.; Elam, J.W.; Darling, S.B.; Shevchenko, E.V. Sequential Infiltration Synthesis for the Design of Low Refractive Index Surface Coatings with Controllable Thickness. ACS Nano 2017. [Google Scholar] [CrossRef]
- Ji, S.; Liu, C.-C.; Son, J.G.; Gotrik, K.; Craig, G.S.W.; Gopalan, P.; Himpsel, F.J.; Char, K.; Nealey, P.F. Generalization of the Use of Random Copolymers To Control the Wetting Behavior of Block Copolymer Films. Macromolecules 2008, 41, 9098–9103. [Google Scholar] [CrossRef]
- Brassat, K.; Kool, D.; Nallet, C.G.A.; Lindner, J.K.N. Understanding Film Thickness-Dependent Block Copolymer Self-Assembly by Controlled Polymer Dewetting on Prepatterned Surfaces. Adv. Mater. Interfaces 2020, 7, 1901605. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Epps, T.H. Self-Assembly of Block Copolymer Thin Films. Mater. Today 2010, 13, 24–33. [Google Scholar] [CrossRef]
- Han, E.; In, I.; Park, S.-M.; La, Y.-H.; Wang, Y.; Nealey, P.F.; Gopalan, P. Photopatternable Imaging Layers for Controlling Block Copolymer Microdomain Orientation. Adv. Mater. 2007, 19, 4448–4452. [Google Scholar] [CrossRef]
- Kim, E.; Ahn, H.; Park, S.; Lee, H.; Lee, M.; Lee, S.; Kim, T.; Kwak, E.-A.; Lee, J.H.; Lei, X.; et al. Directed Assembly of High Molecular Weight Block Copolymers: Highly Ordered Line Patterns of Perpendicularly Oriented Lamellae with Large Periods. ACS Nano 2013, 7, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jun, T.; Lee, W.; Ryu, D.Y. Ordering and Orientation of Giant Nanostructures from High-Molecular-Weight Block Copolymer via Solvent Vapor Annealing Process. J. Photopolym. Sci. Technol. 2018, 31, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent Vapor Annealing of Block Polymer Thin Films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Lundy, R.; Flynn, S.P.; Cummins, C.; Kelleher, S.M.; Collins, M.N.; Dalton, E.; Daniels, S.; Morris, M.A.; Enright, R. Controlled Solvent Vapor Annealing of a High χ Block Copolymer Thin Film. Phys. Chem. Chem. Phys. 2017, 19, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Kim, D.H.; Knoll, W.; Xuan, Y.; Li, B.; Han, Y. Morphologies in Solvent-Annealed Thin Films of Symmetric Diblock Copolymer. J. Chem. Phys. 2006, 125, 064702. [Google Scholar] [CrossRef]
- Xuan, Y.; Peng, J.; Cui, L.; Wang, H.; Li, B.; Han, Y. Morphology Development of Ultrathin Symmetric Diblock Copolymer Film via Solvent Vapor Treatment. Macromolecules 2004, 37, 7301–7307. [Google Scholar] [CrossRef]
- Williams, K.R.; Gupta, K.; Wasilik, M. Etch Rates for Micromachining Processing-Part II. J. Microelectromech. Syst. 2003, 12, 761–778. [Google Scholar] [CrossRef] [Green Version]
- Oehrlein, G.S.; Zhang, Y.; Vender, D.; Haverlag, M. Fluorocarbon High-density Plasmas. I. Fluorocarbon Film Deposition and Etching Using CF4 and CHF3. J. Vac. Sci. Technol. A 1994, 12, 323–332. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barick, B.K.; Shomrat, N.; Green, U.; Katzman, Z.; Segal-Peretz, T. Fabrication of Nanoscale Oxide Textured Surfaces on Polymers. Polymers 2021, 13, 2209. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132209
Barick BK, Shomrat N, Green U, Katzman Z, Segal-Peretz T. Fabrication of Nanoscale Oxide Textured Surfaces on Polymers. Polymers. 2021; 13(13):2209. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132209
Chicago/Turabian StyleBarick, Barun K., Neta Shomrat, Uri Green, Zohar Katzman, and Tamar Segal-Peretz. 2021. "Fabrication of Nanoscale Oxide Textured Surfaces on Polymers" Polymers 13, no. 13: 2209. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132209