1. Introduction
The population growth and economic development have led to an increase in the production and consumption of materials and resources to satisfy global demand. However, this has also led to an increase in the amount of waste produced, such as municipal solid waste, industrial waste, and wastepaper [
1,
2]. Instead of being discarded after primary use, these residues can be used for the production of value-added products e.g., production of low-cost biofuels [
3,
4]. Among wastepaper, great attention has been paid to waste banknotes since they cannot be recycled due to their properties, such as the presence of pathogenic bacteria, heavy ink, and presence of formaldehyde melamine resin that provides protection against humidity and makes the digestion process more challenging [
5,
6,
7,
8].
The most common handling options for banknote paper are incineration or landfilling [
9]. However, these strategies increase air pollution, and at the same time, pollute soils, and groundwater [
10]. As a result, there is a search for more suitable and environmentally friendly handling options that can be applied to waste banknote paper. Banknote paper is to a large extent made of cotton and thus, has a high percentage of cellulose and low amounts of lignin in its composition, which makes it a suitable feedstock for the production of biofuels [
7]. As banknote paper has high amounts of ink in its composition, a deinking process is required before its further treatment. The deinking process is composed of three sequential steps: pulping, soaking, and screening [
7]. In the pulping stage, chemical and mechanical methods are used to separate ink and other non-fibrous contaminants from the fibrous material [
7,
11,
12]. In the soaking stage, sodium hydroxide is used at high temperatures, followed by sieving and rinsing to separate and remove ink particles from the pulp [
13]. Finally, in the screening process, a centrifugal cleaning is used to select and separate contaminants from the pulp fibers. Banknote paper has high amounts of alpha-cellulose in its composition (88 to 96%) which makes it a suitable feedstock for bioethanol and biogas production. However, banknote paper has also a very crystalline structure that requires effective and harsh pretreatments methods to make the cellulose accessible for enzymatic hydrolysis [
7,
14].
Bioethanol production is composed of four sequential steps: pretreatment, hydrolysis, fermentation, and distillation [
15]. A wide variety of chemical, physical, biological, and physio-chemical pretreatment methods have been reported in the literature as effective in breaking down the plant cell wall and in making cellulose accessible to the enzymes [
16]. Chemical pretreatment methods include acid, alkali, ionic liquids, and organosolv. Although chemical pretreatment methods have been widely used and are effective in dissolving lignin and hemicellulose, they are still expensive because of the high cost of chemicals [
17]. Besides, several inhibitors, such as aliphatic carboxylic acids, phenolic compounds, and furans, are formed during chemical pretreatment, which may disrupt subsequent hydrolysis and fermentation processes [
18,
19]. Physical pretreatment methods include comminuting, irradiation, and freezing, while biological pretreatment methods use fungi, enzymes, or microorganisms to break down the lignin and hemicellulose bonds [
14,
16]. Combined physical and chemical pretreatment methods include ammonia fiber expansion, steam explosion (SE), carbon dioxide explosion (CO
2 explosion, and NED).
From all the pretreatment methods reported before, great attention has been paid to chemical-free pretreatment methods (e.g., SE, CO
2 explosion and NED) since these methods reduce environmental impacts and costs that incur when chemicals are used [
20]. Steam explosion pretreatment method uses high pressure, saturated steam, and a rapid decompression to disrupt the plant cell wall and dissolve the hemicellulose [
16]. CO
2 explosion uses supercritical CO
2 and low pretreatment temperatures to improve the digestibility of the biomass [
14]. NED is one of the most effective physical and chemical pretreatments methods reported in the literature [
21]. This pretreatment method used nitrogen and high temperatures to open the biomass structure more effectively. NED is economically and environmentally attractive because neither catalysts nor chemicals are used in the processes [
20]. However, the principle of operation and the processes that take place during this pretreatment still need further research [
19].
Waste paper, and particularly waste banknote paper can also be used as a raw material for biogas production since it is a cheap source of organic material [
22]. Anaerobic digestion is a biological process that occurs in the absence of oxygen and uses micro-organisms to convert organic waste into high-quality nutrient-rich fertilizer and yield energy in the form of biogas [
23]. Biogas is mainly composed of methane (50–70%) and carbon dioxide (30–50%). Minor compounds include vapor water, nitrogen, oxygen, hydrogen sulfide, and ammonia [
24]. Biogas can be upgraded to produce biomethane.
This study aims to investigate the potential of waste banknote paper (with and without ink) for bioethanol and biomethane production. For this purpose, two different pretreatment methods were applied—nitrogen explosive decompression and sulfuric acid pretreatment methods.
2. Materials and Methods
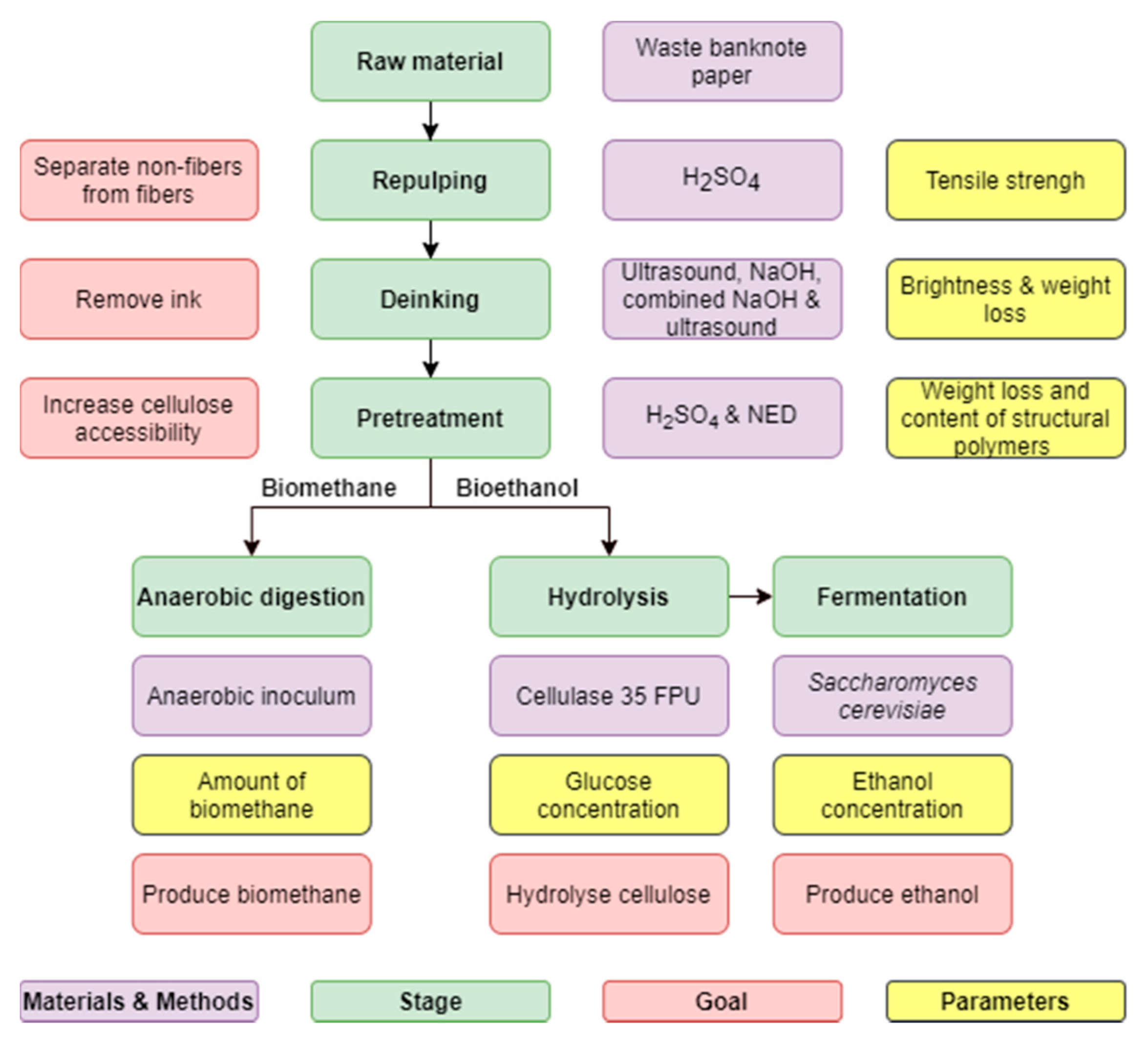
The production pathway utilized in this study to evaluate the potential of waste banknote paper for bioethanol and biogas production is illustrated in
Figure 1.
2.1. Raw Material
Waste banknote paper obtained from the Central Bank of the Islamic Republic of Iran was used as a raw material in this study. After collection, the waste banknote paper was placed in the laboratory for 48 h to ensure equilibrium moisture content. After measuring the moisture content, the raw material was stored in bags until use.
2.2. Repulping
Wet strength is one of the main factors that should be taken into account when talking about waste banknote paper. This property of the paper is related to the presence of melamine formaldehyde, which is added to the banknote paper to increase its mechanical resistance and reduce water absorption. Therefore, the repulping process is required to break down melamine resin cross-links. In order to find the best conditions for the repulping process, the tensile strength was measured at different pHs (from pH = 1 to pH = 12). The lowest tensile strength indicates the best repulping conditions. The tensile strength was measured with a tensile tester, from FRANK-PTI GMBH company (Birkenau, Germany), following the standard method TAPPI T456 OM-15 [
25]. For repulping, 0.5 mL of 98% sulfuric acid was added to 30 g of pulp, and distilled water was added to a 500 mL Erlenmeyer flask until a total working volume of 300 mL was reached. The samples were placed on a hot plate with a magnetic stirrer (M TOPS MS300HS, from Misung Scientific Co., Ltd., Gyeonggi-do, Korea) at 90 °C to 95 °C, and stirred at 500 rpm, for 90 min [
26,
27]. All the experiments were performed in triplicate.
2.3. Deinking
After repulping, the samples were deinked using three deinking methods: chemical (1%, 2%, 3%, and 4% sodium hydroxide), ultrasonic (30Am, for 5 min) and integrated ultrasonic and sodium hydroxide. In the ultrasonic deinking method, a Q700 Sonicator was used (from Qsonica, Newtown, USA). In the deinking process, 3g (dry weight) of pulp was put in 100 mL Erlenmeyer flasks and treated at a temperature of 90–95 °C. To increase the contact during the process, a mixer model FTDS 41 from Sci Finetech Co (Seoul, Korea) was used for 90 min, at 500 rpm. In order to separate and remove the ink from the fibrous materials, the samples were washed using distilled water and a 200 mesh filter. After finishing the deinking process, a new paper was produced, for the weight loss, tensile strength, and brightness measurements. The brightness properties of the papers were measured according to the standard T452 om 08 using a Zb-a Powders brightness colorimeter testing from Hangzhou Zhibang Automation Technology Co. Ltd. (Hangzhou, China) [
7,
26]. The sodium hydroxide with a purity of 100% was from Merck Group (Darmstadt, Germany).
2.4. Pretreatment
2.4.1. Chemical Pretreatment
H2SO4 with 98% purity was used for the chemical pretreatment of the banknote pulp samples. The acid was added in different concentrations (1%, 2%, 3%, and 4%) to Erlenmeyer flasks with 100g (dry weight) deinked waste banknote pulp (WPD) and non-de-inked waste banknote pulp (WPB). The samples were autoclaved for 30 min at 121 °C. After the pretreatment, solid and liquid fractions of the samples were separated by centrifugation (Thermo Scientific Heraeus megacentrifuge, Waltham, USA) at 10,000 rpm for 30 min. Samples from the solid fraction were left to dry at the atmospheric pressure and their weight loss was calculated.
2.4.2. Physio-Chemical Pretreatment
In NED pretreatment, 800 mL of distilled water was added to 100 g of dried WPD and WPB. The samples were mixed and pretreated at 150 °C, 170 °C, 190 °C, and 200 °C, at a pressure of 30 bars using compressed nitrogen gas. After reaching the desired temperatures, the samples were cooled down to 80 °C and the pressure was released from the vessel in an explosive manner [
28]. After the pretreatment, solid and liquid fractions of the samples were separated by centrifugation (Thermo Scientific Heraeus megacentrifuge, Waltham, USA) at 10,000 rpm for 30 min.
2.5. Enzymatic Hydrolysis
Pretreated WPD and WPB samples from the solid fraction were used to make a broth with 2.5% dry matter content that was further used for the enzymatic hydrolysis. The enzyme complex Accellerase 1500 (from DuPont de Nemours) was used at a ratio of 0.3 mL per g of biomass. Distilled water was added to the flasks to obtain a total working volume of 200 mL. The hydrolysis took place for 72 h, at a temperature of 50 °C, under constant stirring in the orbital shaker (IKA
®-Werke GmbH & Co. KG, Staufen im Breisgau, Germany) [
18]. After the hydrolysis, the solid and liquid fractions of the samples were separated by centrifugation (Thermo Scientific Heraeus megacentrifuge, Waltham, USA) at 10,000 rpm for 30 min. All the experiments were performed in triplicate.
2.6. Fermentation
Liquid samples that were obtained from the hydrolysis stage were further fermented using the yeast
Saccharomyces cerevisiae. This yeast was added to the samples from the liquid fraction at the ratio of 0.025 g/g. The fermentation process lasted 7 days and it was carried out at 25 °C [
20].
2.7. Biomethane Potential
The biomethane potential (BMP) test used in this article is based on an adapted version of the protocol reported by Angelidaki et al. [
29]. The inoculum was obtained from Tartu municipal wastewater treatment plant (Estonia), sieved through 2 mm mesh and pre-incubated for 4 days at 36 °C for degasification, before use [
24]. The experiments were carried out in 575 mL plasma bottles with a working volume of 300 mL using the substrate to inoculum volatile solids (VS) ratio of 0.25. The blank test (just inoculum without substrate) was included to study the biogas and methane production of the inoculum, which later was subtracted from that of the samples with the substrate. The test bottles were flushed with N
2 to assure anaerobic conditions. The BMP tests were performed at 36 °C in the lab incubator (Memmert GmbH + Co. KG, Schwabach, Germany) for 32 days (up until the methane content was constant). The experiments were carried out in triplicate. During BMP test the pressure in the headspace of test bottles was measured with pressure meter BMP-Testsystem WAL (WAL Mess- und Regelsysteme GmbH).
2.8. Chemical Analysis
The neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) were determined using an ANKOM 2000 I, fiber Analyzer (ANKOM Technology Corporation, NY 14502, USA).
The dry matter content (TS) was analyzed with a moisture analyzer Ohaus MB 45. The volatile solids (VS) were analyzed as loss on ignition at 550 °C. The pH of the samples before and after pretreatment, hydrolysis, and fermentation was measured using a pH meter, model SevenCompact pH/Ion S220 from Mettler-Toledo AG (Schwerzenbach, Switzerland).
Glucose, glycerol, acetic acid, and ethanol were quantified by HPLC (LC-2030C Plus, Shimadzu, Kyoto, Japan) equipped with a refractive index detector (RID-20A, Shimadzu, Kyoto, Japan) using a Rezex ROA Organic Acid column (Phenomenex, Torrance, CA, USA) column at 45 °C, and isocratic elution at 0.6 mL/min of 5 mmol/L H2SO4.
The quantification of methane in the produced biogas was done by gas chromatography (CP-4900 Micro-GC, from Varian Inc., Palo Alto, USA).
2.9. Calculations
The hydrolysis and fermentation efficiency were calculated based on Equation 1 and Equation (2), respectively [
30].
where E
HY is the hydrolysis efficiency, m
glc the amount of glucose, m
cel, the amount of cellulose, 1.11 is the conversion factor of cellulose to glucose, E
F is the fermentation efficiency, C
eth is the concentration of ethanol, c
glc is the concentration of glucose, and 0.51 is the conversion factor of glucose to ethanol
The methane production was fitted and modelled in the software Graph-Pad Prism 5.0 with a non-linear regression model, using the equation Gompertz growth (Equation (3)).
where Y is the cumulative methane produced (L/kg TS), Y
M is the maximum population (L/kg TS), Y
0 is the starting population (L/kg TS), K is the lag time (d
−1), X is time (days).
2.10. Statistical Analysis
The statistical analysis was performed in software Graph-Pad Prism 5.0. The normal distribution of the results was investigated using the Shapiro-Wilk normality test. For glucose and ethanol yields, the differences between the variables were studied using two-way ANOVA, followed by the post hoc test Tukey’s multiple comparisons test. For biomethane yields, one-way ANOVA was used to investigate the statistically significant differences between the means of the different variables. The post hoc Dunn’s multiple comparisons test. The results were statistically significant when p ≤ 0.05 (confidence interval 95%).
5. Conclusions
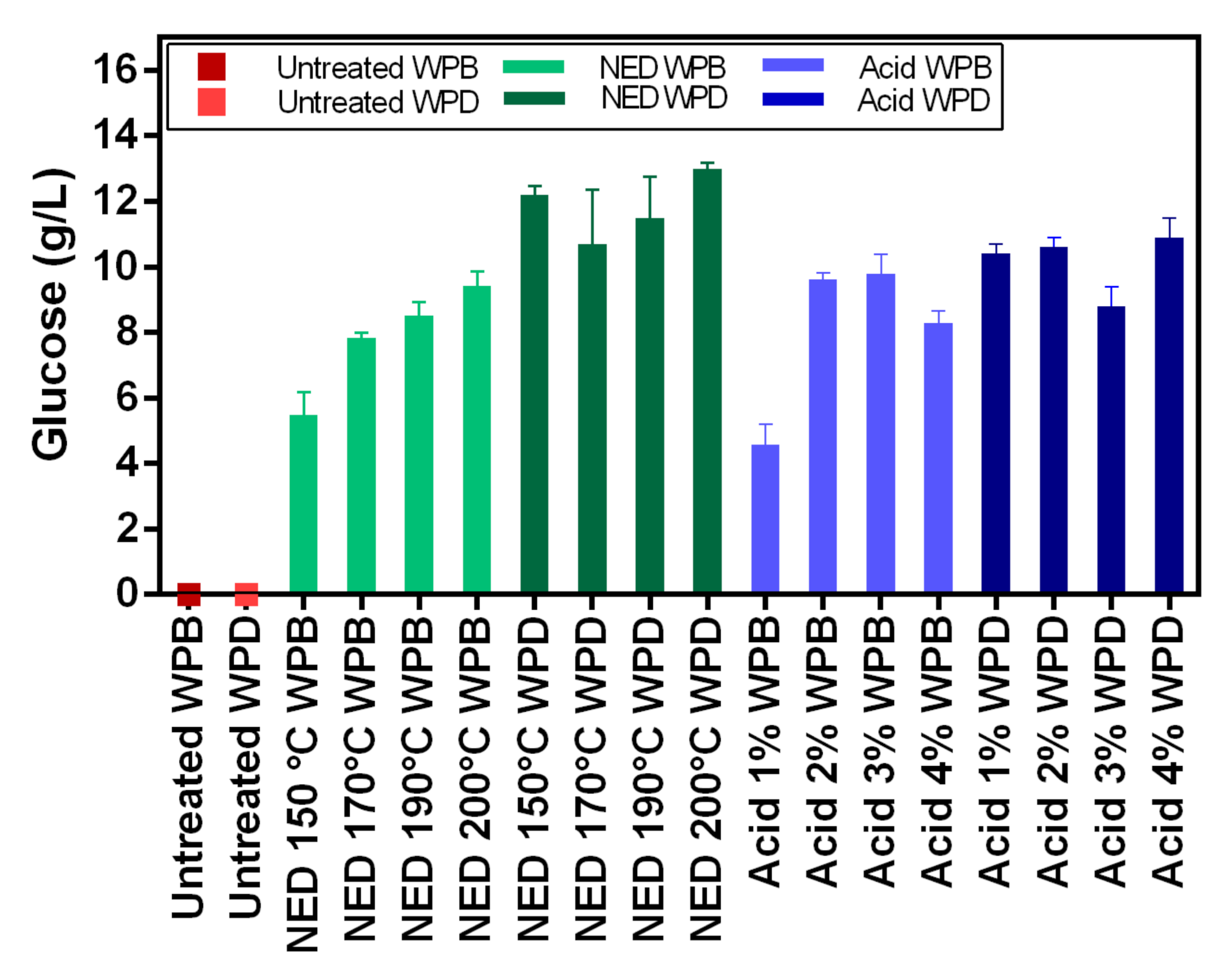
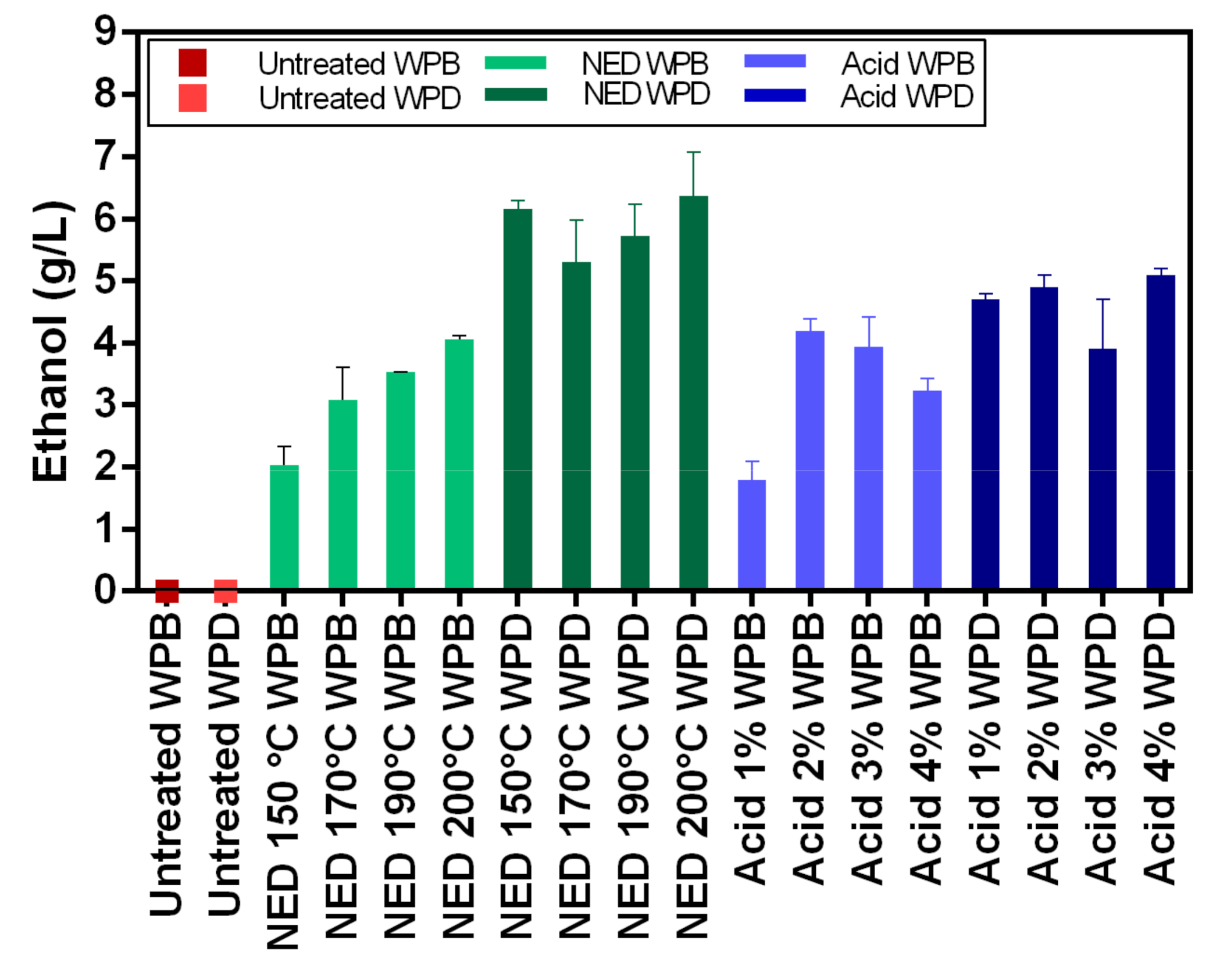
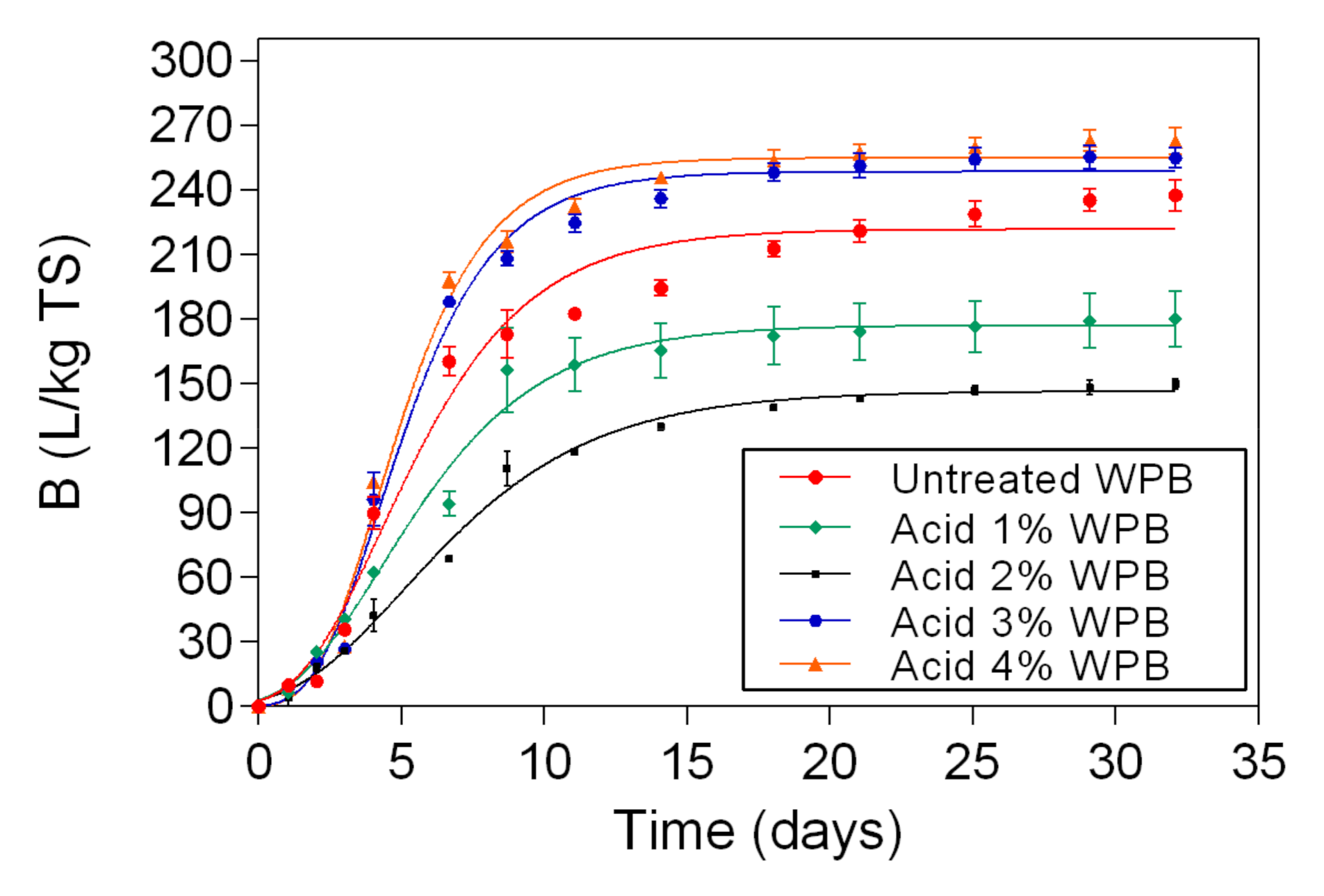
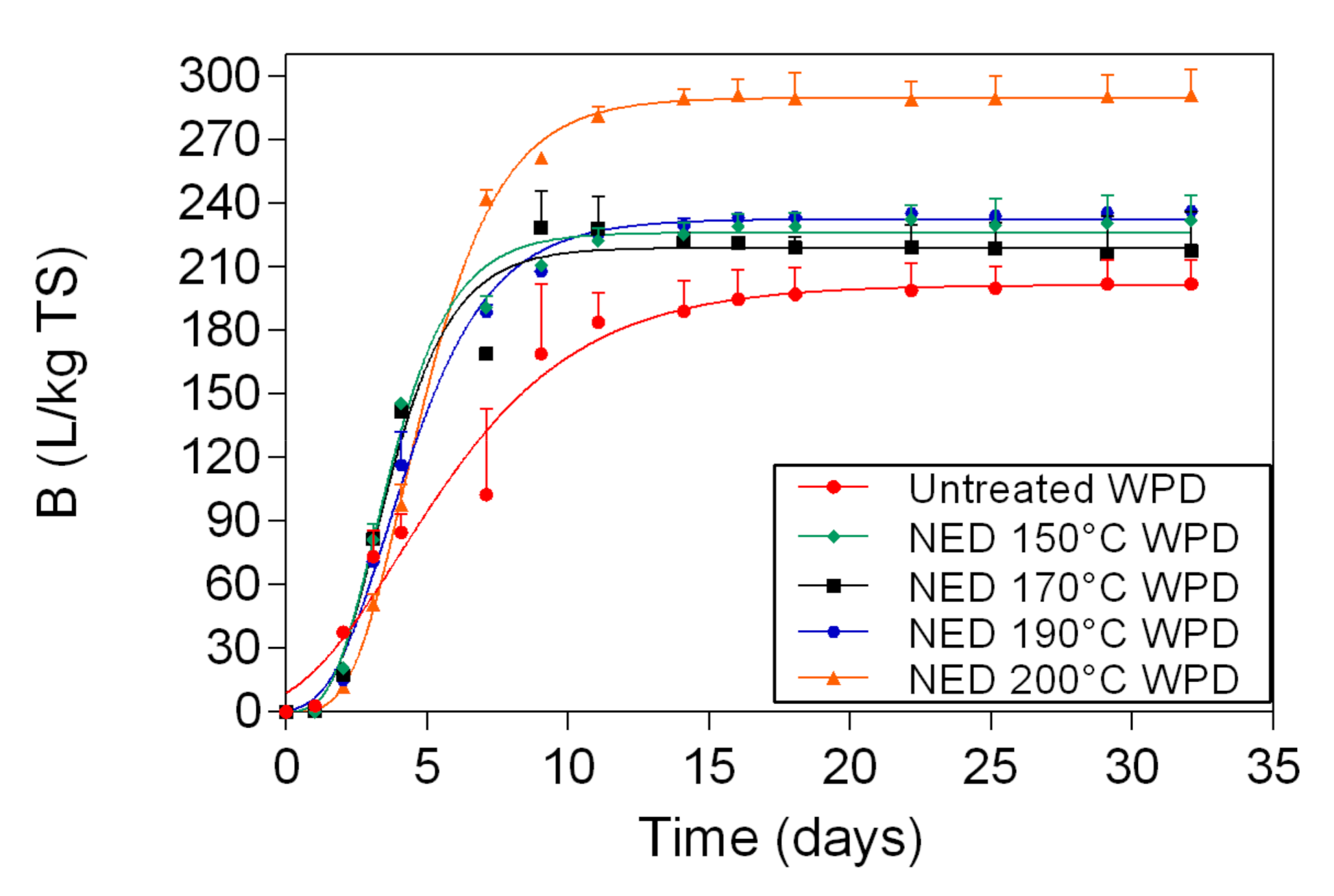
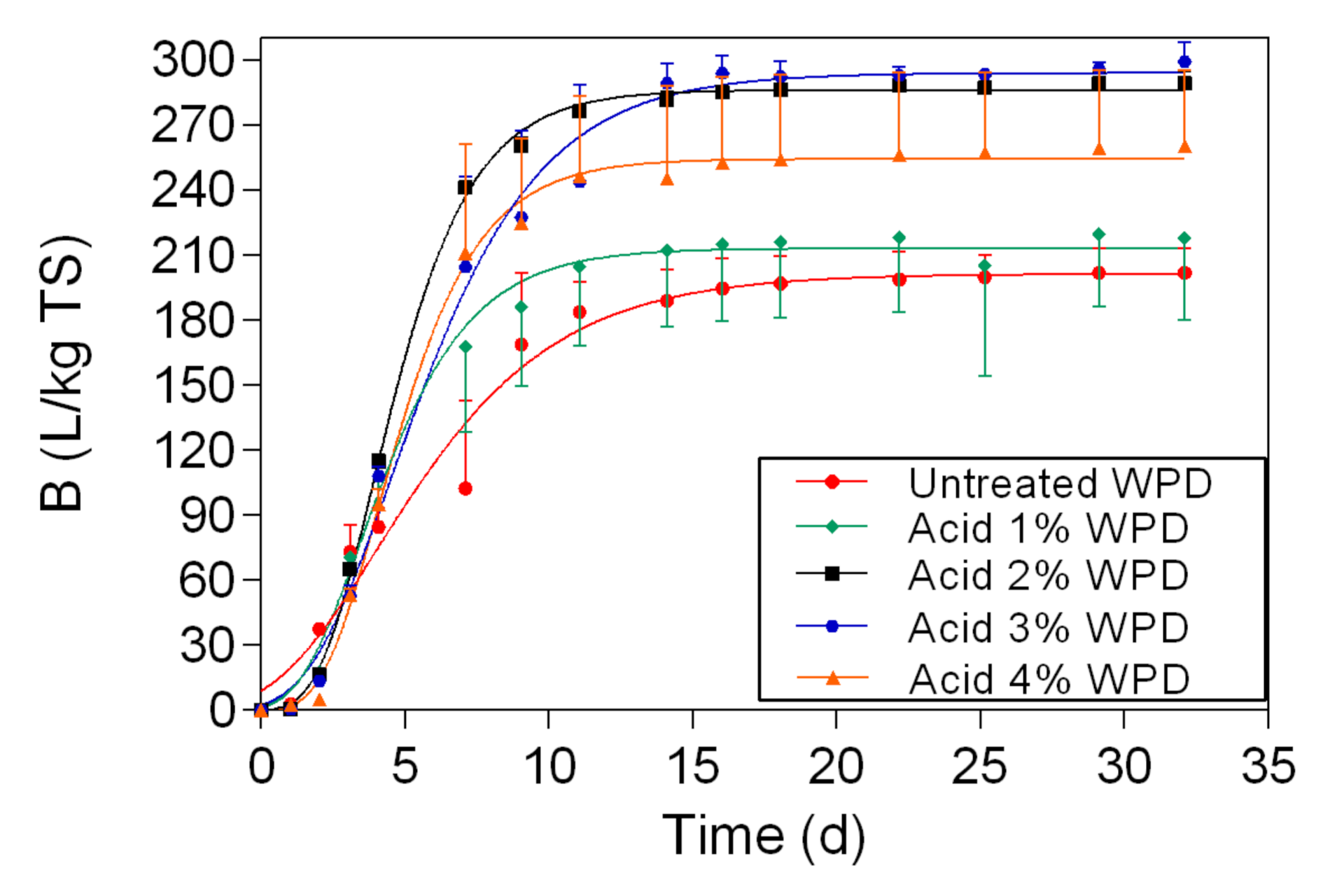
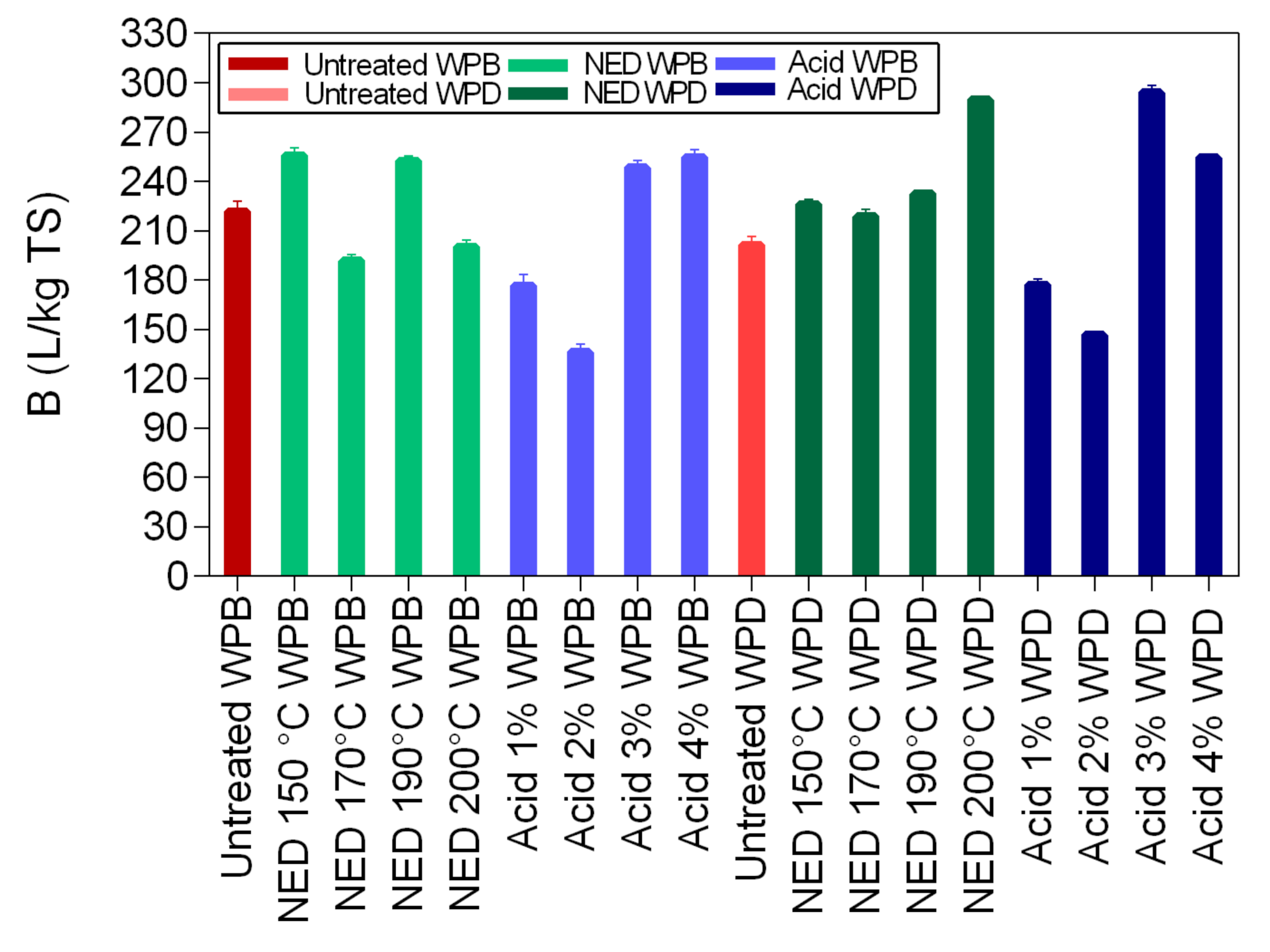
In this study, two different pretreatment methods (chemical and physio-chemical) were used to investigate the potential of waste banknote paper with and without ink for bioethanol and biomethane production. The results of this study show that glucose and ethanol concentrations are higher in WPD samples than for WPB. The glucose yields varied between 4.57 g/L (1% acid WPB) and 12.98 g/L (NED 200 °C WPD), while ethanol yields varied between 1.8 g/L (1% Acid WPB) and 6.36 g/L (NED 200 °C WPD). For WPB samples, the highest biomethane yields were reported in samples that were pretreated with NED 150 °C (266 L/kg TS) and 4% acid (263 L/Kg TS). For WPD samples, samples that were pretreated with 3% acid (299 L/Kg TS) and NED 200 °C (292 L/Kg TS) pretreatments insured the highest biomethane production. In general, the biomethane potential of WPD samples was higher than that of similarly pretreated WPB samples. NED pretreatment method gives higher glucose, ethanol, and biomethane yields than samples that were pretreated with sulfuric acid. The deinking process, the acid concentration, and the pretreatment temperature all influence glucose, bioethanol, and biomethane yields. More research should be done to find the most efficient ways to increase glucose, bioethanol, and biomethane yields.