The Double-Faced Electrostatic Behavior of PNIPAm Microgels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microgel-Polyion Complexes
2.3. Viscosimetry
2.4. Light Scattering and Electrophoretic Measurements
2.5. Microscopy
3. Results
3.1. Characterization of Bare Microgels
3.2. Polyelectrolyte-Microgel Suspensions
- (i)
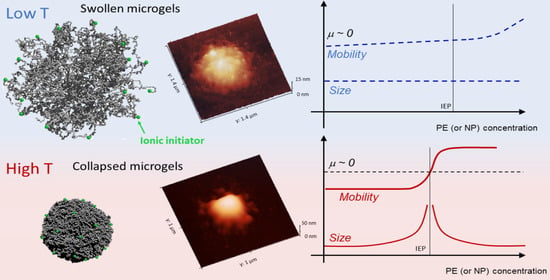
- Though we observe systematically the crossover from negative to positive average mobilities for all temperatures, the modalities with which such a variation occurs change qualitatively when passing from to . Below mobility smoothly increases, without showing a sudden jump from negative to positive values and suggests that PLL chains adsorb onto PNIPAm microgels even when the latter are swollen. Above the mobility passes from highly negative to highly positive values in a very narrow range of PLL concentrations. This is what is expected when ion-ion correlation increases in the Debye layer in proximity of an adsorption surface. In fact, based on a modified Poisson equation, proposed by Bazant and coworkers [80] to capture the effect of ion-ion correlation in equilibrium Debye layers, Stout and Khair [81] showed recently that the extent of electrophoretic mobility reversal and its sharpness in colloid-multivalent ion systems are both enhanced by an increase of the ion-ion correlation length. In the case of PNIPAm microgels with tunable charge density we attribute this increased ion-ion correlation to the enhanced adsorption energy on the microgel periphery. Moreover, the authors [81] find that the point where vanishes does not correspond exactly to the point of zero charge, and that the mobility reversal occurs at progressively lower ion concentration for increasing correlation length among the ions. As a matter of fact, when a colloid is immersed in a large multivalent ion solution a large contribution to the net force exerted on the colloid is given by the electro-osmotic hydrodynamic force, constantly directed in opposite direction to the applied electric field.Both electric and electro-osmotic force decrease in magnitude as the colloid is progressively neutralized by spatially correlated polyions, but the rate of this decrease differs for the two forces as the correlation length characterizing adsorbed polyion is large enough. For such a reason a still negatively charged decorated colloid can move in the electric field direction and hence with a reversed mobility and the original sign of the charge. Though the different porosity and electrolyte diffusion time scales characterizing swollen and collapsed microgels should be considered to exhaustively capture the physics ruling the microgel electrophoretic behavior [82], this overall scenario conforms to our polyelectrolyte-microgel complexes, where polyion correlation is intensified by an increase of the adsorption energy due to microgel collapse. This said, for our systems the isoelectric point, classically identified as the point where , does not lie far from the true isoelectric condition, where the net charge of the complex is zero. As we will show later in this section, large aggregates form and flocculation occurs in proximity of the mobility reversal, where thus the net charge of decorated microgels must be low and the interaction among them dominated by charge patch attractions.
- (ii)
- The PLL concentration at which the average mobility reversal occurs sharply drops at (data reported in Figure 6 and further discussed later in this section).
3.3. Nanoparticle-Microgel Suspensions
3.4. Thermal Reversibility of Overcharged Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Nieves, A.; Wyss, H.M.; Mattsson, J.; Weitz, D.A. (Eds.) Microgel Suspensions, Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Pich, A.; Richtering, W. (Eds.) Chemical Design of Responsive Microgels; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; Volume 234. [Google Scholar]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Karg, M.; Hellweg, T. New “smart” poly(NIPAM) microgels and nanoparticle microgel hybrids: Properties and advances in characterisation. Curr. Opin. Colloid Interface Sci. 2009, 14, 438–450. [Google Scholar] [CrossRef]
- Weng, H.; Zhou, J.; Tang, L.; Hu, Z. Tissue responses to thermally-responsive hydrogel nanoparticles. J. Biomater. Sci. Polym. Ed. 2004, 15, 1167–1180. [Google Scholar] [CrossRef]
- Wang, J.; Gan, D.; Lyon, L.A.; El-Sayed, M.A. Temperature-Jump Investigations of the Kinetics of Hydrogel Nanoparticle Volume Phase Transitions. J. Am. Chem. Soc. 2001, 123, 11284–11289. [Google Scholar] [CrossRef]
- Reese, C.E.; Mikhonin, A.V.; Kamenjicki, M.; Tikhonov, A.; Asher, S.A. Nanogel Nanosecond Photonic Crystal Optical Switching. J. Am. Chem. Soc. 2004, 126, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermoresponsive polymers as novel biomaterials. Macromol. Biosci. 2006, 6, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Minko, S.; Eichhorn, K.J.; Nitschke, M.; Simon, F.; Stamm, M. Reversible tuning of wetting behavior of polymer surface with responsive polymer brushes. Langmuir 2003, 19, 8077–8085. [Google Scholar] [CrossRef]
- Szabo, D.; Szeghy, G.; Zrinyi, M. Shape transition of magnetic field sensitive polymer gels. Macromolecules 1998, 31, 6541–6548. [Google Scholar] [CrossRef]
- Siegel, R.; Firestone, B. pH-dependent equilibrium swelling properties of hydrophobic polyelectrolyte copolymer gels. Macromolecules 1988, 21, 3254–3259. [Google Scholar] [CrossRef]
- Sutani, K.; Kaetsu, I.; Uchida, K. The synthesis and the electric-responsiveness of hydrogels entrapping natural polyelectrolyte. Radiat. Phys. Chem. 2001, 61, 49–54. [Google Scholar] [CrossRef]
- Guan, T.; Godts, F.; Ceyssens, F.; Vanderleyden, E.; Adesanya, K.; Dubruel, P.; Neves, H.; Puers, R. Development and fabrication of a novel photopatternable electric responsive Pluronic hydrogel for MEMS applications. Sens. Actuators A 2012, 186, 184–190. [Google Scholar] [CrossRef]
- ter Schiphorst, J.; Coleman, S.; Stumpel, J.; Azouz, A.; Diamond, D.; Schenning, J.A. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem. Mater. 2015, 27, 5925–5931. [Google Scholar] [CrossRef] [Green Version]
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.; Ionov, L. 4D biofabrication using shape-morphing hydrogels. Adv. Mater 2017, 29, 1703443. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kondo, M.; Miyasato, R.; Naka, Y.; Mamiya, J.I.; Kinoshita, M.; Shishido, A.; Yu, Y.; Barrett, C.; Ikeda, T. Photomobile polymer materials—Various three-dimensional movements. J. Mater. Chem. 2009, 19, 60–62. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Zhang, L.; Cao, X. 4D printing of robust hydrogels consisted of agarose nanofibers and polyacrylamide. ACS Macro Lett. 2018, 7, 442–446. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Yu, H. Endowing soft photo-actuators with intelligence. Adv. Intell. Syst. 2019, 1, 1900050. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Pei, Z.; Li, Z.; Wei, Y.; Ji, Y. Making and remaking dynamic 3D structures by shining light on flat liquid crystalline vitrimer films without a mold. J. Am. Chem. Soc. 2016, 138, 2118–2121. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.; Yu, Y.; Kinoshita, M.; Barrett, C.; Ikeda, T. Photomobile polymer materials: Towards light-driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef]
- Richtering, W.; Alberg, I.; Zentel, R. Nanoparticles in the Biological Context: Surface Morphology and Protein Corona Formation. Small 2020, 16, 2002162. [Google Scholar] [CrossRef] [PubMed]
- Crassous, J.J.; Mihut, A.M.; Månsson, L.K.; Schurtenberger, P. Anisotropic responsive microgels with tuneable shape and interactions. Nanoscale 2015, 7, 15971–15982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cors, M.; Wiehemeier, L.; Hertle, Y.; Feoktystov, A.; Cousin, F.; Hellweg, T.; Oberdisse, J. Structure and position-dependent properties of inhomogeneous suspensions of responsive colloids. Langmuir 2018, 34, 15403–15415. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.A.d.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.J.; Gnan, N.; Obiols-Rabasa, M.; Meijer, J.M.; Rovigatti, L.; Zaccarelli, E.; Schurtenberger, P. A new look at effective interactions between microgel particles. Nat. Commun. 2018, 9, 5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippe, A.M.; Truzzolillo, D.; Galvan-Myoshi, J.; Dieudonné-George, P.; Trappe, V.; Berthier, L.; Cipelletti, L. Glass transition of soft colloids. Phys. Rev. E 2018, 97, 040601. [Google Scholar] [CrossRef] [Green Version]
- Picard, C.; Garrigue, P.; Tatry, M.C.; Lapeyre, V.; Ravaine, S.; Schmitt, V.; Ravaine, V. Organization of Microgels at the Air–Water Interface under Compression: Role of Electrostatics and Cross-Linking Density. Langmuir 2017, 33, 7968–7981. [Google Scholar] [CrossRef]
- Pelton, R.H.; Pelton, H.M.; Morphesis, A.; Rowell, R.L. Particle Sizes and Electrophoretic Mobilities of Poly (N-Isopropylacrylamide) Latex. Langmuir 1989, 5, 816–818. [Google Scholar] [CrossRef]
- Braibanti, M.; Haro-Pérez, C.; Quesada-Pérez, M.; Rojas-Ochoa, L.F.; Trappe, V. Impact of Volume Transition on the Net Charge of Poly- N -Isopropyl Acrylamide Microgels. Phys. Rev. E 2016, 94, 032601. [Google Scholar] [CrossRef] [Green Version]
- Truzzolillo, D.; Sennato, S.; Sarti, S.; Casciardi, S.; Bazzoni, C.; Bordi, F. Overcharging and reentrant condensation of thermoresponsive ionic microgels. Soft Matter 2018, 14, 4110–4125. [Google Scholar] [CrossRef] [Green Version]
- Bordi, F.; Sennato, S.; Truzzolillo, D. Polyelectrolyte-Induced Aggregation of Liposomes: A New Cluster Phase with Interesting Applications. J. Phys. Condens. Matter 2009, 21, 203102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kimura, K.; Dubin, P.L.; Jaeger, W. Polyelectrolyte-Micelle Coacervation: Effects of Micelle Surface Charge Density, Polymer Molecular Weight, and Polymer/Surfactant Ratio. Macromolecules 2000, 33, 3324–3331. [Google Scholar] [CrossRef]
- Gillies, G.; Lin, W.; Borkovec, M. Charging and Aggregation of Positively Charged Latex Particles in the Presence of Anionic Polyelectrolytes. J. Phys. Chem. B 2007, 111, 8626–8633. [Google Scholar] [CrossRef] [PubMed]
- Keren, K.; Soen, Y.; Yoseph, G.B.; Gilad, R.; Braun, E.; Sivan, U.; Talmon, Y. Microscopics of Complexation between Long DNA Molecules and Positively Charged Colloids. Phys. Rev. Lett. 2002, 89, 088103. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, V.A.; Sergeyev, V.G.; Pyshkina, O.A.; Zinchenko, A.A.; Zezin, A.B.; Joosten, J.G.H.; Brackman, J.; Yoshikawa, K. Interpolyelectrolyte Complexes Formed by DNA and Astramol Poly(propylene imine) Dendrimers. Macromolecules 2000, 33, 9587–9593. [Google Scholar] [CrossRef]
- Milkova, V.; Kamburova, K.; Petkanchin, I.; Radeva, T. Complexation of Ferric Oxide Particles with Pectins of Different Charge Density. Langmuir 2008, 24, 9495–9499. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Sennato, S.; Diociaiuti, M. Direct Evidence of Multicompartment Aggregates in Polyelectrolyte-Charged Liposome Complexes. Biophys. J. 2006, 91, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Volodkin, D.; Ball, V.; Schaaf, P.; Voegel, J.C.; Mohwald, H. Complexation of phosphocholine liposomes with polylysine. Stabilization by surface coverage versus aggregation. Biochim. Biophys. Acta Biomembr. 2007, 1768, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Radler, J.; Koltover, I.; Jamieson, A.; Salditt, T.; Safinya, R. Structure and Interfacial Aspects of Self-AssembledCationic Lipid-DNA Gene Carrier Complexes. Langmuir 1998, 14, 4272–4283. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Marianecci, C.; Carafa, M.; Cametti, M. Hybrid Niosome Complexation in the Presence of Oppositely Charged Polyions. J. Phys. Chem. B 2008, 112, 3720–3727. [Google Scholar] [CrossRef]
- Lu, P.J.; Conrad, J.C.; Wyss, H.M.; Schofield, A.B.; Weitz, D.A. Fluids of Clusters in Attractive Colloids. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Polotsky, A.A.; Plamper, F.A.; Borisov, O.V. Collapse-to-Swelling Transitions in pH- and Thermoresponsive Microgels in Aqueous Dispersions: The Thermodynamic Theory. Macromolecules 2013, 46, 8702–8709. [Google Scholar] [CrossRef]
- Greinert, N.; Richtering, W. Influence of polyelectrolyte multilayer adsorption on the temperature sensitivity of poly(N-isopropylacrylamide) (PNiPAM) microgels. Colloid Polym. Sci. 2004, 282, 1146–1149. [Google Scholar] [CrossRef]
- Wong, J.E.; Díez-Pascual, A.M.; Richtering, W. Layer-by-Layer Assembly of Polyelectrolyte Multilayers on Thermoresponsive P(NiPAM- co -MAA) Microgel: Effect of Ionic Strength and Molecular Weight. Macromolecules 2009, 42, 1229–1238. [Google Scholar] [CrossRef]
- Chen, R.; Jin, X.; Zhu, X. Investigation of the Formation Process of PNIPAM-Based Ionic Microgels. ACS Omega 2017, 2, 8788–8793. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, Y.; Zhang, M.; Zhang, H. Initiator Systems Effect on Particle Coagulation and Particle Size Distribution in One-Step Emulsion Polymerization of Styrene. Polymers 2016, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wei, J.; Ngai, T.; Wang, L.; Zhu, D.; Shen, J. Correlation between Dielectric/Electric Properties and Cross-Linking/Charge Density Distributions of Thermally Sensitive Spherical PNIPAM Microgels. Macromolecules 2012, 45, 6158–6167. [Google Scholar] [CrossRef]
- Hiraki, J. ε-Polylysine, its development and utilization. Fine Chem. 2000, 29, 18–25. [Google Scholar]
- Hamano, Y.; Kito, N.; Kita, A.; Imokawa, Y.; Yamanaka, K.; Maruyama, C.; Katano, H. ε-Poly-L-Lysine Peptide Chain Length Regulated by the Linkers Connecting the Transmembrane Domains of ε-Poly-L-Lysine Synthetase. Appl. Environ. Microbiol. 2014, 80, 4993–5000. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Nagasawa, T. ε-Poly-l-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The Antimicrobial Mechanism of Action of Epsilon-Poly-l-Lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, W.J.; Foster, J.F. Influence of Helix Content and Solvent Environment on the Optical Rotatory Dispersion Parameters of Polypeptides. Mol. Biol. 1963, 7, 590–598. [Google Scholar] [CrossRef]
- Noguchi, H. Studies of the helix-coil transition of poly-L-lysine in film and solution. Biopolymers 1966, 4, 1105–1113. [Google Scholar] [CrossRef]
- Davidson, B.; Fasmann, D.G. The Conformational Transitions of Uncharged Poly-L-lysine. α-Helix-Random Coil-β Structure. Biochemistry 1967, 6, 1616–1629. [Google Scholar]
- Ciferri, A.; Puett, D.; Rajagh, L.; Mermans, J.J. Potentiometric Titrations and the Helix-Coil Transition of Poly (L-glutamic Acid) and Poly-Llysine in Aqueous Salt Solutions. Biopolymers 1968, 8, 1019–1036. [Google Scholar] [CrossRef]
- Myer, Y. The pH-Induced Helix-Coil Transition of Poly-L-lysine and Poly-L-glutamic Acid and the 238-mμ Dichroic Band. Macromolecules 1969, 2, 624–628. [Google Scholar] [CrossRef]
- Greenfield, N.; Fasman, D.G. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef]
- Liem, R.; Poland, D.; Scheraga, H. Titration of α-helical poly-L-lysine in 95% methanol. A Study of the Range of the Electrostatic Potential in Polypeptides. J. Am. Chem. Soc. 1970, 92, 5717–5724. [Google Scholar] [CrossRef]
- Pederson, D.; Gabriel, D.; Hermans, J.J. Potentiometric titration of poly-L-lysine: The coil-to-β transition. Biopolymers 1971, 10, 2133–2145. [Google Scholar] [CrossRef]
- Shepherd, I. Study of poly-L-lysine conformations in aqueous methanol solution by using polarized Raman techniques. Biochem. J. 1976, 155, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.; Haris, P.; Chapman, D. Conformational transitions in poly(l-lysine): Studies using Fourier transform infrared spectroscopy. Biochim. Biophys. Acta 1989, 998, 75–79. [Google Scholar] [CrossRef]
- Vorobjev, Y.; Scheraga, H.; Honig, B. Theoretical Modeling of Electrostatic Effects of Titratable Side Chain Groups on Protein Conformation in a Polar Ionic Solution. 2. pH-Induced Helix-Coil Transition of Poly(L-lysine) in Water and Methanol Ionic Solutions. J. Phys. Chem. 1995, 99, 7180–7187. [Google Scholar] [CrossRef]
- Dzwolak, W.; Muraki, T.; Kato, M.; Taniguchi, Y. Chain-length dependence of alpha-helix to beta-sheet transition in polylysine: Model of protein aggregation studied by temperature-tuned FTIR spectroscopy. Biopolymers 2004, 73, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, D.; Mathur, K.; Balasubramanian, D. Poly(ϵ-L-lysine): Synthesis and conformation. Biopolymers 1980, 19, 219–229. [Google Scholar] [CrossRef]
- Maeda, S.; Kunimoto, K.; Sasaki, C.; Kuwae, A.; Hanai, K. Characterization of microbial poly (ϵ-L-lysine) by FT-IR, Raman and solid state 13C NMR spectroscopies. J. Mol. Struct. 2003, 655, 149–155. [Google Scholar] [CrossRef]
- Asano, A.; Tanaka, C.; Murata, Y. NMR determination of crystallinity for poly(epsilon-L-lysine). Polymer 2007, 48, 3809–3816. [Google Scholar] [CrossRef]
- Jia, S.; Fan, B.; Dai, Y.; Wang, G.; Peng, P.; Jia, Y. Fractionation and characterization of ϵ-poly-L-lysine from Streptomyces albulus CGMCC 1986. Food Sci. Biotechnol. 2010, 19, 361–366. [Google Scholar] [CrossRef]
- Truzzolillo, D.; Roger, V.; Dupas, C.; Mora, S.; Cipelletti, L. Bulk and Interfacial Stresses in Suspensions of Soft and Hard Colloids. J. Phys. Condens. Matter 2015, 27, 194103. [Google Scholar] [CrossRef] [Green Version]
- Sennato, S.; Carlini, L.; Truzzolillo, D.; Bordi, F. Salt-Induced Reentrant Stability of Polyion-Decorated Particles with Tunable Surface Charge Density. Colloids Surf. B Biointerfaces 2016, 137, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Romeo, G.; Fernandez-Nieves, A.; Wyss, H.M.; Acierno, D.; Weitz, D.A. Temperature-Controlled Transitions Between Glass, Liquid, and Gel States in Dense p-NIPA Suspensions. Adv. Mater. 2010, 22, 3441–3445. [Google Scholar] [CrossRef]
- Dhadwal, H.S.; Ansari, R.R.; Meyer, W.V. A Fiber-optic Probe for Particle Sizing in Concentrated Suspensions. Rev. Sci. Instrum. 1991, 62, 2963–2968. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Provencher, S.W. A Constrained Regularization Method for Inverting Data Represented by Linear Algebraic or Integral Equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Tscharnuter, W.W. Mobility Measurements by Phase Analysis. Appl. Opt. 2001, 40, 3995–4003. [Google Scholar] [CrossRef]
- Maki, Y.; Sugawara, K.; Nagai, D. Temperature Dependence of Electrophoretic Mobility and Hydrodynamic Radius of Microgels of Poly(N-isopropylacrylamide). Gels 2018, 4, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, E.; Saunders, B.R. Temperature–dependent Electrophoretic Mobility and Hydrodynamic Radius Measurements of Poly(N-Isopropylacrylamide) Microgel Particles: Structural Insights. Phys. Chem. Chem. Phys. 2000, 2, 3187–3193. [Google Scholar] [CrossRef]
- Tagit, O.; Tomczak, N.; Vancso, G.J. Probing the Morphology and Nanoscale Mechanics of Single Poly(N-isopropylacrylamide) Microgels Across the Lower-Critical-Solution Temperature by Atomic Force Microscopy. Small 2008, 4, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bazant, M.Z.; Storey, B.D.; Kornyshev, A.A. Double Layer in Ionic Liquids: Overscreening versus Crowding. Phys. Rev. Lett. 2011, 106, 046102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, R.F.; Khair, A.S. A continuum approach to predicting electrophoretic mobility reversals. J. Fluid Mech. 2014, 752. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Li, M.; Bresson, B.; Cousin, F.; Fretigny, C.; Tran, Y. Submicrometric Films of Surface-Attached Polymer Network with Temperature-Responsive Properties. Langmuir 2015, 31, 11516–11524. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chang, Y.C. Synthesis and Conformational Transition of Surface-Tethered Polypeptide: Poly(L-lysine). Macromolecules 2003, 36, 6511–6518. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Deshkovski, A.; Rubinstein, M. Adsorption of Polyelectrolytes at Oppositely Charged Surfaces. Macromolecules 2001, 34, 3421–3436. [Google Scholar] [CrossRef]
- Jin, X.; Leclercq, L.; Sisavath, N.; Cottet, H. Investigating the Influence of Phosphate Ions on Poly(L-lysine) Conformations by Taylor Dispersion Analysis. Macromolecules 2014, 47, 5320–5327. [Google Scholar] [CrossRef]
- Brant, D.A.; Flory, P.J. The Configuration of Random Polypeptide Chains. I. Experimental Results. J. Am. Chem. Soc. 1965, 87, 2788–2791. [Google Scholar] [CrossRef]
- Varenne, F.; Coty, J.B.; Botton, J.; Legrand, F.X.; Hillaireau, H.; Barratt, G.; Vauthier, C. Evaluation of zeta potential of nanomaterials by electrophoretic light scattering: Fast field reversal versus Slow field reversal modes. Talanta 2019, 205, 120062. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Z.; Xu, Z.; Masliyah, J. Bitumen–Clay Interactions in Aqueous Media Studied by Zeta Potential Distribution Measurement. J. Colloid Interface Sci. 2002, 252, 409–418. [Google Scholar] [CrossRef]
- Liang, L.; Wang, L.; Nguyen, A.V.; Xie, G. Heterocoagulation of alumina and quartz studied by zeta potential distribution and particle size distribution measurements. Powder Technol. 2017, 309, 1–12. [Google Scholar] [CrossRef] [Green Version]













| Sample Code | Crosslinker/NIPAm Molar Ratio | Initiator/NIPAm Molar Ratio |
|---|---|---|
| m1-KPS | 0.013 | 0.016 |
| m5-KPS | 0.052 | 0.016 |
| m5-AIBA | 0.054 | 0.010 |
| Sample Code | [C] | [C] | [C] | [C] | ||[μm cm/Vs] |
|---|---|---|---|---|---|
| m1-KPS | |||||
| m5-KPS | |||||
| m5-AIBA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sennato, S.; Chauveau, E.; Casciardi, S.; Bordi, F.; Truzzolillo, D. The Double-Faced Electrostatic Behavior of PNIPAm Microgels. Polymers 2021, 13, 1153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071153
Sennato S, Chauveau E, Casciardi S, Bordi F, Truzzolillo D. The Double-Faced Electrostatic Behavior of PNIPAm Microgels. Polymers. 2021; 13(7):1153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071153
Chicago/Turabian StyleSennato, Simona, Edouard Chauveau, Stefano Casciardi, Federico Bordi, and Domenico Truzzolillo. 2021. "The Double-Faced Electrostatic Behavior of PNIPAm Microgels" Polymers 13, no. 7: 1153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071153