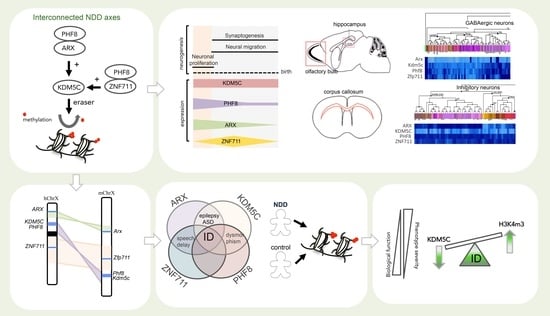
Analysis of a Set of KDM5C Regulatory Genes Mutated in Neurodevelopmental Disorders Identifies Temporal Coexpression Brain Signatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Identification of Cap Analysis of Gene Expression (CAGE) Derived Transcription Start Sites
2.3. Cell Lines, Transient Transfection, and Luciferase Assay
2.4. Plasmids
2.5. Western Blotting and Protein Interaction Assays
2.6. RNA Extraction and Real-Time Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. Spatiotemporal Expression Patterns of KDM5C and Its Regulatory Genes in Human and Mouse Brain
3.2. CAGE-Defined TSSs of KDM5C and Its Regulatory Genes in Human and Mouse Brain
3.3. KDM5C, ARX, PHF8, and ZNF711 Are Syntenic Genes of Human/Mouse X-Chromosome
3.4. ARX Interacts with PHF8
3.5. Analysis of KDM5C and H3K4me3 Levels in NDD Patient-Derived Lymphoblastoid Cell Lines
3.6. The Defective ARX-Dependent Transactivation of KDM5C Is Balanced by PHF8 or ZNF711 Overexpression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meaney, M.J.; Ferguson-Smith, A. Epigenetic regulation of the neural transcriptome: The meaning of the marks. Nat. Neurosci. 2010, 13, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Mossink, B.; Negwer, M.; Schubert, D.; Kasri, N.N. The emerging role of chromatin remodelers in neurodevelopmental disorders: A developmental perspective. Cell. Mol. Life Sci. 2021, 78, 2517–2563. [Google Scholar] [CrossRef] [PubMed]
- Ciptasari, U.; Van Bokhoven, H. The phenomenal epigenome in neurodevelopmental disorders. Hum. Mol. Genet. 2020, 29, R42–R50. [Google Scholar] [CrossRef]
- Poeta, L.; Fusco, F.; Drongitis, D.; Shoubridge, C.; Manganelli, G.; Filosa, S.; Paciolla, M.; Courtney, M.; Collombat, P.; Lioi, M.B.; et al. A Regulatory Path Associated with X-Linked Intellectual Disability and Epilepsy Links KDM5C to the Polyalanine Expansions in ARX. Am. J. Hum. Genet. 2013, 92, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Poeta, L.; Padula, A.; Attianese, B.; Valentino, M.; Verrillo, L.; Filosa, S.; Shoubridge, C.; Barra, A.; Schwartz, C.E.; Christensen, J.; et al. Histone demethylase KDM5C is a SAHA-sensitive central hub at the crossroads of transcriptional axes involved in multiple neurodevelopmental disorders. Hum. Mol. Genet. 2019, 28, 4089–4102. [Google Scholar] [CrossRef]
- Shen, E.; Shulha, H.P.; Weng, Z.; Akbarian, S. Regulation of histone H3K4 methylation in brain development and disease. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130514. [Google Scholar] [CrossRef]
- Iwase, S.; Lan, F.; Bayliss, P.; De La Torre-Ubieta, L.; Huarte, M.; Qi, H.; Whetstine, J.R.; Bonni, A.; Roberts, T.M.; Shi, Y. The X-Linked Mental Retardation Gene SMCX/JARID1C Defines a Family of Histone H3 Lysine 4 Demethylases. Cell 2007, 128, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Scandaglia, M.; Lopez-Atalaya, J.P.; Medrano-Fernandez, A.; Lopez-Cascales, M.T.; del Blanco, B.; Lipinski, M.; Benito, E.; Olivares, R.; Iwase, S.; Shi, Y.; et al. Loss of Kdm5c Causes Spurious Transcription and Prevents the Fine-Tuning of Activity-Regulated Enhancers in Neurons. Cell Rep. 2017, 21, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallianatos, C.N.; Raines, B.; Porter, R.S.; Bonefas, K.M.; Wu, M.C.; Garay, P.M.; Collette, K.M.; Seo, Y.A.; Dou, Y.; Keegan, C.E.; et al. Mutually suppressive roles of KMT2A and KDM5C in behaviour, neuronal structure, and histone H3K4 methylation. Commun. Biol. 2020, 3, 278. [Google Scholar] [CrossRef]
- Jensen, L.R.; Amende, M.; Gurok, U.; Moser, B.; Gimmel, V.; Tzschach, A.; Janecke, A.; Tariverdian, G.; Chelly, J.; Fryns, J.-P.; et al. Mutations in the JARID1C Gene, Which Is Involved in Transcriptional Regulation and Chromatin Remodeling, Cause X-Linked Mental Retardation. Am. J. Hum. Genet. 2005, 76, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Abidi, F.E.; Holloway, L.; Moore, C.A.; Weaver, D.D.; Simensen, R.J.; Stevenson, R.E.; Rogers, R.C.; Schwartz, C.E. Mutations in JARID1C are associated with X-linked mental retardation, short stature and hyperreflexia. J. Med. Genet. 2008, 45, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Vallianatos, C.N.; Farrehi, C.; Friez, M.J.; Burmeister, M.; Keegan, C.E.; Iwase, S. Altered Gene-Regulatory Function of KDM5C by a Novel Mutation Associated with Autism and Intellectual Disability. Front. Mol. Neurosci. 2018, 11, 104. [Google Scholar] [CrossRef]
- Adegbola, A.; Gao, H.; Sommer, S.; Browning, M. A novel mutation inJARID1C/SMCX in a patient with autism spectrum disorder (ASD). Am. J. Med Genet. Part A 2008, 146A, 505–511. [Google Scholar] [CrossRef]
- Brookes, E.; Laurent, B.; Ounap, K.; Carroll, R.; Moeschler, J.B.; Field, M.; Schwartz, C.E.; Gecz, J.; Shi, Y. Mutations in the intellectual disability gene KDM5C reduce protein stability and demethylase activity. Hum. Mol. Genet. 2015, 24, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Shoubridge, C.; Fullston, T.; Gecz, J. ARX spectrum disorders: Making inroads into the molecular pathology. Hum. Mutat. 2010, 31, 889–900. [Google Scholar] [CrossRef]
- Kato, M.; Das, S.; Petras, K.; Kitamura, K.; Morohashi, K.-I.; Abuelo, D.N.; Barr, M.; Bonneau, D.; Brady, A.F.; Carpenter, N.J.; et al. Mutations ofARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum. Mutat. 2004, 23, 147–159. [Google Scholar] [CrossRef]
- Kato, M.; Saitoh, S.; Kamei, A.; Shiraishi, H.; Ueda, Y.; Akasaka, M.; Tohyama, J.; Akasaka, N.; Hayasaka, K. A Longer Polyalanine Expansion Mutation in the ARX Gene Causes Early Infantile Epileptic Encephalopathy with Suppression-Burst Pattern (Ohtahara Syndrome). Am. J. Hum. Genet. 2007, 81, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laperuta, C.; Spizzichino, L.; D’Adamo, P.; Monfregola, J.; Maiorino, A.; D’Eustacchio, A.; Ventruto, V.; Neri, G.; D’Urso, M.; Chiurazzi, P.; et al. MRX87 family with Aristaless Xdup24bp mutation and implication for polyAlanine expansions. BMC Med. Genet. 2007, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Chaste, P.; Nygren, G.; Anckarsäter, H.; Råstam, M.; Coleman, M.; Leboyer, M.; Gillberg, C.; Betancur, C. Mutation screening of theARX gene in patients with autism. Am. J. Med. Genet. Part B: Neuropsychiatr. Genet. 2007, 144B, 228–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poeta, L.; Drongitis, D.; Verrillo, L.; Miano, M.G. DNA Hypermethylation and Unstable Repeat Diseases: A Paradigm of Transcriptional Silencing to Decipher the Basis of Pathogenic Mechanisms. Genes 2020, 11, 684. [Google Scholar] [CrossRef]
- Nasrallah, M.P.; Cho, G.; Simonet, J.C.; Putt, M.E.; Kitamura, K.; Golden, J.A. Differential effects of a polyalanine tract expansion in Arx on neural development and gene expression. Hum. Mol. Genet. 2011, 21, 1090–1098. [Google Scholar] [CrossRef]
- Mattiske, T.; Lee, K.; Gecz, J.; Friocourt, G.; Shoubridge, C. Embryonic forebrain transcriptome of mice with polyalanine expansion mutations in theARXhomeobox gene. Hum. Mol. Genet. 2016, 25, 5433–5443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumonnier, F.; Holbert, S.; Ronce, N.; Faravelli, F.; Lenzner, S.; Schwartz, C.E.; Lespinasse, J.; Van Esch, H.; Lacombe, D.; Goizet, C.; et al. Mutations in PHF8 are associated with X linked mental retardation and cleft lip/cleft palate. J. Med. Genet. 2005, 42, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidi, F.E.; Miano, M.G.; Murray, J.C.; Schwartz, C.E. A novel mutation in the PHF8 gene is associated with X-linked mental retardation with cleft lip/cleft palate. Clin. Genet. 2007, 72, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Zhou, Y.; Han, Y.; Li, S.; Xu, Q.; Xu, L.; Zhu, Z.; Deng, Y.; Yu, L.; et al. Phf8 histone demethylase deficiency causes cognitive impairments through the mTOR pathway. Nat. Commun. 2018, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Perez, A.A.; Schreiner, S.; Nicolet, C.M.; Farnham, P.J. Characterization of the ZFX family of transcription factors that bind downstream of the start site of CpG island promoters. Nucleic Acids Res. 2020, 48, 5986–6000. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Smith, R.; Pleasance, E.; Whibley, A.; Edkins, S.; Hardy, C.; O’Meara, S.; Latimer, C.; Dicks, E.; Menzies, A.; et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 2009, 41, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kantojärvi, K.; Kotala, I.; Rehnström, K.; Ylisaukko-Oja, T.; Vanhala, R.; Von Wendt, T.N.; Von Wendt, L.; Jarvela, I. Fine mapping of Xq11.1-q21.33 and mutation screening of RPS6KA6, ZNF711, ACSL4, DLG3, and IL1RAPL2 for autism spectrum disorders (ASD). Autism Res. 2011, 4, 228–233. [Google Scholar] [CrossRef]
- Van der Werf, I.M.; Van Dijck, A.; Reyniers, E.; Helsmoortel, C.; Kumar, A.; Kalscheuer, V.M.; de Brouwer, A.P.; Kleefstra, T.; van Bokhoven, H.; Mortier, G.; et al. Mutations in two large pedigrees highlight the role of ZNF711 in X-linked intellectual disability. Gene 2017, 605, 92–98. [Google Scholar] [CrossRef]
- Ropers, H.-H.; Hamel, B.C.J. X-linked mental retardation. Nat. Rev. Genet. 2005, 6, 46–57. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Leushkin, E.; Liechti, A.; Ovchinnikova, S.; Mößinger, K.; Brüning, T.; Rummel, C.; Grützner, F.; Cardoso-Moreira, M.; Janich, P.; et al. Transcriptome and translatome co-evolution in mammals. Nat. Cell Biol. 2020, 588, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Vitezic, M.; Bertin, N.; Andersson, R.; Lipovich, L.; Kawaji, H.; Lassmann, T.; Sandelin, A.; Heutink, P.; Goldowitz, D.; Ha, T.; et al. CAGE-defined promoter regions of the genes implicated in Rett Syndrome. BMC Genom. 2014, 15, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchelli, S.; Fedele, S.; Vatta, P.; Calligaris, R.; Heutink, P.; Rizzu, P.; Itoh, M.; Persichetti, F.; Santoro, C.; Kawaji, H.; et al. Antisense Transcription in Loci Associated to Hereditary Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 5392–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleine-Kohlbrecher, D.; Christensen, J.; Vandamme, J.; Abarrategui, I.; Bak, M.; Tommerup, N.; Shi, X.; Gozani, O.; Rappsilber, J.; Salcini, A.E.; et al. A Functional Link between the Histone Demethylase PHF8 and the Transcription Factor ZNF711 in X-Linked Mental Retardation. Mol. Cell 2010, 38, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-temporal transcriptome of the human brain. Nat. Cell Biol. 2011, 478, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Fassio, A.; Patry, L.; Congia, S.; Onofri, F.; Piton, A.; Gauthier, J.; Pozzi, D.; Messa, M.; Defranchi, E.; Fadda, M.; et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum. Mol. Genet. 2011, 20, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melloni, R.; Apostolides, P.; Hamos, J.; DeGennaro, L. Dynamics of synapsin I gene expression during the establishment and restoration of functional synapses in the rat hippocampus. Neuroscience 1994, 58, 683–703. [Google Scholar] [CrossRef]
- Tahiliani, M.; Mei, P.; Fang, R.; Leonor, T.; Rutenberg, M.; Shimizu, F.; Li, J.; Rao, A.; Shi, Y. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nat. Cell Biol. 2007, 447, 601–605. [Google Scholar] [CrossRef] [PubMed]
- FANTOM Consortium and the RIKEN PMI and CLST; Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Purmann, A.; Toedling, J.; Schueler, M.; Carninci, P.; Lehrach, H.; Hayashizaki, Y.; Huber, W.; Sperling, S. Genomic organization of transcriptomes in mammals: Coregulation and cofunctionality. Genomics 2007, 89, 580–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienvenu, T.; Poirier, K.; Friocourt, G.; Bahi, N.; Beaumont, D.; Fauchereau, F.; Ben Jeema, L.; Zemni, R.; Vinet, M.-C.; Francis, F.; et al. ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum. Mol. Genet. 2002, 11, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Curie, A.; Nazir, T.; Brun, A.; Paulignan, Y.; Reboul, A.; Delange, K.; Cheylus, A.; Bertrand, S.; Rochefort, F.; Bussy, G.; et al. The c.429_452 duplication of the ARX gene: A unique developmental-model of limb kinetic apraxia. Orphanet J. Rare Dis. 2014, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.R.; Bartenschlager, H.; Rujirabanjerd, S.; Tzschach, A.; Nümann, A.; Janecke, A.R.; Spörle, R.; Stricker, S.; Raynaud, M.; Nelson, J.; et al. A distinctive gene expression fingerprint in mentally retarded male patients reflects disease-causing defects in the histone demethylase KDM5C. PathoGenetics 2010, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Haas, S.; Chelly, J.; Van Esch, H.; Raynaud, M.; De Brouwer, A.P.M.; Weinert, S.; Froyen, G.; Frints, S.G.; Laumonnier, F.; et al. X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes. Mol. Psychiatry 2016, 21, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzschach, A.; Lenzner, S.; Moser, B.; Reinhardt, R.; Chelly, J.; Fryns, J.-P.; Kleefstra, T.; Raynaud, M.; Turner, G.; Ropers, H.-H.; et al. NovelJARID1C/SMCX mutations in patients with X-linked mental retardation. Hum. Mutat. 2006, 27, 389. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Alexov, E. Cofactors-loaded quaternary structure of lysine-specific demethylase 5C (KDM5C) protein: Computational model. Proteins Struct. Funct. Bioinform. 2016, 84, 1797–1809. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Cho, S.; Chen, T.; Rosenberg-Lee, M.; Geary, D.C.; Menon, V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 2014, 17, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T.; Moody, J.; Kwon, A.T.-J.; Shibayama, Y.; Kato, S.; Huang, Y.; Böttcher, M.; Motakis, E.; Mendez, M.; Severin, J.; et al. C1 CAGE detects transcription start sites and enhancer activity at single-cell resolution. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Verrillo, L.; Mangano, E.; Drongitis, D.; Merelli, I.; Pischedda, F.; Piccoli, G.; Consolandi, C.; Bordoni, R.; Miano, M.G. A reliable strategy for single-cell RNA sequencing analysis using cryoconserved primary cortical cells. J. Neurosci. Methods 2021, 347, 108960. [Google Scholar] [CrossRef] [PubMed]
- Shoubridge, C.; Tan, M.H.; Fullston, T.; Cloosterman, D.; Coman, D.; McGillivray, G.; Mancini, G.M.; Kleefstra, T.; Gecz, J. Mutations in the nuclear localization sequence of the Aristaless related homeobox; sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution of the transcription factor and retards cell division. PathoGenetics 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Gao, G.; Liu, K.; Shi, X.; Cheng, M.; Xiong, Y.; Song, S. Projections from D2 Neurons in Different Subregions of Nucleus Accumbens Shell to Ventral Pallidum Play Distinct Roles in Reward and Aversion. Neurosci. Bull. 2021, 37, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Poirier, K.; Lacombe, D.; Gilbert-Dussardier, B.; Raynaud, M.; Desportes, V.; De Brouwer, A.P.M.; Moraine, C.; Fryns, J.P.; Ropers, H.H.; Beldjord, C.; et al. Screening of ARX in mental retardation families: Consequences for the strategy of molecular diagnosis. Neurogenetics 2005, 7, 39–46. [Google Scholar] [CrossRef] [PubMed]








| Gene | cDNA Change | Protein Change | Mutation | Clinical Features | Reference |
|---|---|---|---|---|---|
| ARX | c.298_330dup33 | p.Ala105_115dup | duplication | DEE1, severe ID | [4,18,42,43] |
| c.429_452dup24 | p.Ala148_155dup | duplication | mild ID | [4,18,42,43] | |
| KDM5C | c.1162G>C | p.Ala388Pro | missense | ID, mild dysmorphism | [10] |
| c.3864G>A | p.Trp1288X | nonsense | severe ID, spasticity, epilepsy, microcephaly | [10,44] | |
| c.1599delC | p.Trp534Glyfs*15 | nonsense | severe ID, epilepsy, spasticity | [45] | |
| c.2248C>T | p.Arg750Trp | missense | severe ID, speech impairment | [46] | |
| PHF8 | c.1050_1061del12 | p.Pro314fs* | nonsense | mild to borderline ID, cleft lip and cleft palate | [23] |
| ZNF711 | c.731T>C | p.Ile244Thr | missense | mild to borderline ID, speech delay | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poeta, L.; Padula, A.; Lioi, M.B.; van Bokhoven, H.; Miano, M.G. Analysis of a Set of KDM5C Regulatory Genes Mutated in Neurodevelopmental Disorders Identifies Temporal Coexpression Brain Signatures. Genes 2021, 12, 1088. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12071088
Poeta L, Padula A, Lioi MB, van Bokhoven H, Miano MG. Analysis of a Set of KDM5C Regulatory Genes Mutated in Neurodevelopmental Disorders Identifies Temporal Coexpression Brain Signatures. Genes. 2021; 12(7):1088. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12071088
Chicago/Turabian StylePoeta, Loredana, Agnese Padula, Maria Brigida Lioi, Hans van Bokhoven, and Maria Giuseppina Miano. 2021. "Analysis of a Set of KDM5C Regulatory Genes Mutated in Neurodevelopmental Disorders Identifies Temporal Coexpression Brain Signatures" Genes 12, no. 7: 1088. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12071088