1. Introduction
Global warming is gaining wider recognition in the world. Fossil fuel power plants are responsible for approximately one-third of global CO
2 emissions to the atmosphere. Therefore, removing CO
2 from low-pressure flue gas (i.e., CO
2 capture in postcombustion) has been the focus of extensive research over the last few decades. As an alternative to the energy-intensive amine-based chemical absorption processes, CO
2 capture via adsorption on renewable biomass-derived carbons has gained increased interest, since these adsorbents are relatively cheap, require low energy for regeneration, and show a relatively good tolerance to moisture existing in flue gas [
1].
An increasing number of studies have focused on producing activated carbons (ACs) from different biomass precursors (through physical or chemical activation) and assessing their performance in terms of CO
2 uptake at 10–15 kPa and selectivity towards CO
2 over N
2 [
2,
3,
4,
5,
6,
7,
8,
9,
10]. At equilibrium conditions (i.e., adsorption isotherms of pure components), the biomass-derived ACs exhibit a relatively high CO
2 adsorption capacity, usually in the range of 1.0–2.0 mmol g
−1 at 15 kPa and 25 °C [
9]. Regarding the apparent CO
2/N
2 selectivity, which is defined as the ratio of molar uptakes divided by the ratio of partial pressures, values in the range of 8–14 (deduced from single component adsorption data at 25–50 °C) have been reported in previous studies [
6,
11,
12]. It is generally accepted that the narrower micropores of ACs are the main responsible for the physical adsorption of CO
2 at low pressure. Therefore, and for a given AC, the CO
2 uptake at 10–15 kPa is primarily a function of its ultra-micropore volume (pore width below 0.7 nm).
From a more realistic point of view, studies focusing on the behavior of biomass-derived ACs under dynamic conditions are explicitly required, since cyclic breakthrough measurements are more representative of practical separations. Adsorbents have to exhibit relatively fast adsorption and desorption kinetics for their successful implementation in swing adsorption processes. In this sense, the number of studies available is relatively modest compared to the large amount of research already conducted on developing ACs from biomass precursors. In a very interesting study [
13], the performance under dynamic conditions of olive stone and almond shells-derived ACs (produced by single-step activation with CO
2 at 800 °C) was tested. For a feed stream composed of a binary mixture of CO
2/N
2 (14/86 vol. %) at 50 °C and 120 kPa, the authors reported good CO
2 adsorption capacities (around 0.6 mmol g
−1) and apparent selectivities CO
2/N
2 (20–30) for fresh adsorbent. The fact that the selectivity increased for dynamic measurements was mainly explained by a certain decrease in the adsorbed amount of the weaker adsorbate (N
2) in the presence of the stronger one (CO
2). In other words, the apparent CO
2/N
2 selectivity estimated from the single-component adsorption isotherms could be lower than that measured under dynamic conditions for binary mixtures.
Shahkarami et al., [
14] assessed the performance of several agricultural waste-based carbons, which were produced through slow or fast pyrolysis and then activated with KOH. The best results were obtained for a pinewood sawdust-derived AC, which exhibited high CO
2 uptake (1.8 mmol g
−1 after nine cycles) and apparent selectivity CO
2/N
2 (29) at 25 °C and 101.3 kPa (for a binary mixture CO
2/N
2 15/85 vol. %). However, the temperature tested by Shahkarami et al., [
14] was not within the typical range for postcombustion flue gas (40–60 °C). Moreover, the gas residence time selected by the authors (31 s) was relatively long. In this sense, capital costs for large-scale units operating at these conditions could be unacceptable. More representative operating conditions (adsorption temperatures of 45 and 60 °C, and gas residence times of 16–17 s) were considered in the study by Shafeeyan et al., [
15], in which CO
2 adsorption capacities of 0.57 and 0.47 mmol g
−1 (at 45 and 60 °C, respectively) were reported for a granular palm shell-derived N-doped AC.
The three above-mentioned studies, however, were conducted under dry conditions. Given that the real flue gas contains a certain amount of moisture (which is unavoidable in practice), the effect of water vapor on the CO
2 adsorption capacity and CO
2/N
2 selectivity under dynamic conditions should also be assessed. In this regard, there are relatively few experimental studies available in the literature that have explored the performance of biomass-derived ACs under humid conditions. Among them, it is worth mentioning the study by Xu et al., [
16], where the performance of a commercial coconut shell-derived AC was tested during a vacuum swing adsorption (VSA) cycle under humid conditions. In the experiment conducted by Xu et al., the adsorption step was conducted at an absolute pressure of 120 kPa, at a bed temperature of 60 °C, and using a feed gas steam composed of CO
2 (12 vol. %), water (4.8 vol. %), and air (balance). Surprisingly, the authors reported an almost identical recovery and purity of CO
2 in the presence of water as compared to the dry condition. However, this study did not assess the performance of the tested adsorbent over sequential adsorption/desorption cycles.
Durán et al., [
17] investigated the ability of a pine sawdust-based AC to selectively adsorb CO
2 from wet biogas under dynamic conditions (at an absolute pressure of 135 kPa, 30 °C, and using a quaternary gas mixture of CH
4/CO
2/N
2/H
2O
(v) with a relative humidity of 55%). The authors observed that, under humid conditions, the CO
2 uptake and breakthrough time decreased in comparison with those measured during the breakthrough experiments under dry conditions. In fact, the breakthrough time was reduced by a factor of 1.85 for a molar ratio CO
2/CH
4 of 30/70 in the feed gas mixture. However, as a remarkable finding, Durán et al., [
17] stated that the presence of water vapor on the bed can promote the CO
2 adsorption over CH
4, leading to a more efficient separation with respect to the dry case.
The specific aim of the present study is to explore the feasibility of using biomass-derived physically activated biochars as cost-effective adsorbents for CO2 capture from humid postcombustion flue gas. The performance of physically activated carbons based on vine shoots as well as wheat straw pellets was assessed via cyclic breakthrough experiments under dry and severe humid conditions (relative humidity of 100%). Both biomass sources were selected on the basis of their potential sustainability, since they are agricultural wastes (residual biomass) that do not compete with food, feed or timber production either directly or indirectly within a specific area.
2. Materials and Methods
2.1. Biochar-Based Adsorbents
Two types of agricultural wastes were used as carbon precursors: (1) vine shoots (VS) from a local vineyard (which were previously cut into pieces of 1.0–3.5 cm in length), and (2) free-binder wheat straw pellets (WS) from a Belgian company (7 mm OD and approximately 12 mm long). Proximate and ultimate analyses were conducted for both biomass sources (further details on these measurements are given in the
Supplementary Materials).
The biomass feedstocks were pyrolyzed under nitrogen in a packed-bed reactor at a highest temperature (HTT) of 500 and 600 °C for WS and VS, respectively. Two different values of absolute pressures (0.1 and 0.5 MPa) were tested. Details on the pyrolysis device and experimental procedure are available elsewhere [
18,
19]. Briefly, approximately 400 g of raw biomass were heated at an average heating rate of 5 °C min
−1 to the highest temperature with a soaking time of 60 min at this temperature. The volumetric flow rate of the carrier gas (N
2) within the reactor at the highest temperature was kept constant, regardless of the pressure applied, by properly adjusting the mass flow rate. Assuming an entire reactor’s void-volume fraction of 0.9, the above-mentioned flow rate led to a as residence time of the carrier gas within the reactor of 100 s.
The obtained biochars (which were milled and sieved to a particle size distribution of 0.212–1.41 mm) were physically activated with CO
2 at 800 °C in a quartz tubular fixed-bed reactor (ID = 16 mm), which was placed in a vertical tube furnace (model EVA 12/300 from Carbolite Gero, UK). The reactor was filled with biochar at a bed height of 300 mm and then heated at 10 °C min
−1 under a steady flow of N
2 (500 mL min
−1 STP) at atmospheric pressure. Once the bed reached the desired temperature (800 °C), the gas flow was switched from pure N
2 to a mixture of CO
2 and N
2 (in the volume ratio of 20:80) at a total mass flow rate of 500 mL min
−1 STP. Given that the porosity of raw biochars ranged from 0.65 to 0.75, a gas-hourly space velocity (GHSV) of 5600–7800 h
−1 was estimated. A holding time of 1 h was applied in order to obtain a burn-off degree of 20–30%, depending on the reactivity of the given precursor. To properly determine the degree of burn-off, biochars were previously pyrolyzed (under N
2 atmosphere) at a highest temperature of 800 °C.
Table 1 summarizes the preparation procedure for the ACs tested in the present study. The proximate and ultimate analyses of the activated biochars were also performed.
2.2. Static Gas Adsorption Measurements
For textural characterization purposes, N
2 adsorption/desorption isotherms at −196 °C and CO
2 adsorption isotherms at 0 °C were acquired using an ASAP 2020 gas sorption analyzer from Micromeritics (USA). Samples (around 120–180 mg) were previously degassed under dynamic vacuum conditions to constant weight at a temperature of 150 °C. From the N
2 adsorption/desorption isotherms at −196 °C, we estimated the apparent specific surface area (
SBET), micropore volume (
Vmic), mesopore volume (
Vmes), and pore size distribution (PSD) for pore sizes above 0.9–1.0 nm. The data from the CO
2 adsorption isotherms at 0 °C were used to estimate the ultra-micropore volume (
Vultra, for pore sizes lower than 0.7 nm) and the PSD for narrow micropores. Further details regarding the procedures used to estimate the above-mentioned parameters are given in the
Supplementary Material.
The single-component adsorption isotherms (for both CO2 and N2), at temperatures of 25 and 50 °C, on the activated biochars (at pressures ranging from 0 to 101 kPa) were measured using the same above-mentioned device as well as the same degasification procedure.
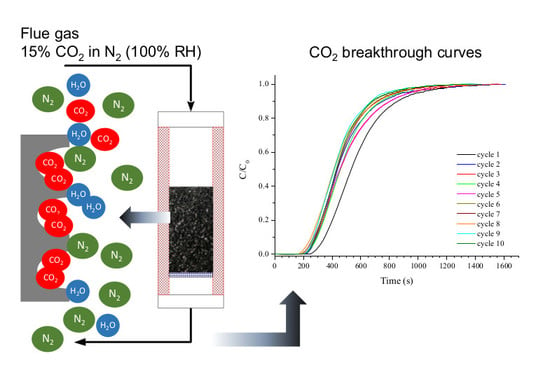
2.3. Dynamic Breakthrough Experiments
Cyclic adsorption-desorption measurements were conducted in a custom-built device, whose schematic diagram is given in
Figure 1. The adsorption column consisted of an AISI 316-L tubular reactor (250 mm length; 20.9 mm ID), which was heated by a PID controlled electric furnace. A K-type thermocouple was placed in the center of the packed bed. Experiments were performed using an initial mass of adsorbent that corresponded to a bed height of 220 mm. Small particle sizes (i.e., below 0.212 mm) were discarded to avoid excessive pressure drop. A small portion of fused quartz wool was placed at both ends of the column to prevent the loss of adsorbent.
The feed flow rates of pure N2, CO2, and helium were adjusted using mass flow controllers (Brooks, USA). A mass flow meter (Omega Engineering, UK) was used to measure the outlet gas flow rate. CO2 concentrations were measured using a continuous gas analyzer (ABB model EL3020, Germany). During the adsorption step, the absolute pressure was kept constant at a value of 110–115 kPa. The desorption step was conducted under vacuum (VSA) using an oil rotary vane vacuum pump (Busch, Germany), which assured an absolute pressure of 10 kPa at suction flow rate up to 0.33 m3 s−1 STP.
In a typical cyclic experiment under dry conditions, the adsorbent was initially outgassed by purging with helium at 150 °C for 4 h. Afterwards, the bed was cooled down to the desired temperature (25 or 50 °C). Adsorption was then started by switching the feeding gas from helium to a dry mixture of N2 and CO2 (with a CO2 composition of 13.75–14.25 vol. %) at a total flow rate of 3.67 cm3 s−1 STP. Under these operating conditions and assuming a void fraction of 0.65–0.75 (estimated from the measured pressure drop across the bed, which ranged from 165 to 430 Pa, and the Ergun equation), the gas residence time within the packed bed was 13.2 ± 1.3 and 12.2 ± 1.2 s at 25 and 50 °C, respectively. Once saturation was reached, the feeding gas was turned off and the column outlet was connected to the vacuum pump. During the desorption step, the temperature of the packed bed was kept at the same value than for the preceding adsorption step.
For the dynamic adsorption measurements under humid conditions (at a temperature of 50 °C), 0.0226 cm3 min−1 of water was added to the dry feeding gas (at the same flow rate as mentioned above) using a HPLC pump (model 521 from Analytical Scientific Instruments, USA). The amount of added water corresponded to a relative humidity (RH) of the wet gas of approximately 100% at the operating conditions of the column. The water content of the wet feeding gas was 11.2 vol. %.
Since the dead volume of the system was relatively large, especially for the experiments conducted under humid conditions (in which the moisture trap and measurement chamber were also used), blank adsorption tests were carried out to estimate the time required to displace the dead volume. The blank tests were conducted in both dry and humid conditions for an empty and filled (with glass beads of 1.0 mm OD) adsorption column.
4. Conclusions
From the results discussed above, we can conclude that, in general, physically activated biochars produced from both wheat straw and vine shoots are promising adsorbents for CO2 capture under realistic postcombustion conditions. As expected, pore size distribution plays a key role in the CO2 adsorption behavior. In this sense, biomass-derived activated carbons having a hierarchical structure (with a high volume of ultra-micropores but also a certain volume of mesopores) appear as the ideal candidates for an efficient and selective CO2 adsorption from a dry flue gas under dynamic conditions. However, the presence of relatively high concentrations of water vapor in the feeding gas clearly interferes with the CO2 adsorption mechanism, leading to significantly shorter breakthrough times (i.e., lower percentages of used bed). The differences observed here in the performance under humid conditions of two activated carbons (produced from different biochar precursors) suggest that surface chemistry, and especially the concentration and accessibility of hydrophilic OFGs, could exert considerable influence on the water adsorption rate. Therefore, future research should focus on synthetizing biomass-derived activated carbons with an appropriate hierarchical pore size distribution, relatively high bulk density, and low affinity to water molecules. For this purpose, the combined effects of the nature of the precursor, pyrolysis conditions, activation conditions, and degree of burn-off should properly be addressed.