Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects and Study Design
2.2. Sample Size Calculation
2.3. Animal Model and Groups
2.4. Nanostructured Carbonated Hydroxyapatite (nCHA) Synthesis
2.5. Preparation of Biomaterial for Grafting
2.6. Anesthetic and Surgical Procedures
2.7. Histological Process
2.8. Image Acquisition by SR-µCT and 3D Segmentation and Processing
2.9. Histological Analysis
2.10. Histomorphometric Evaluation
2.11. Statistical Analysis
3. Results
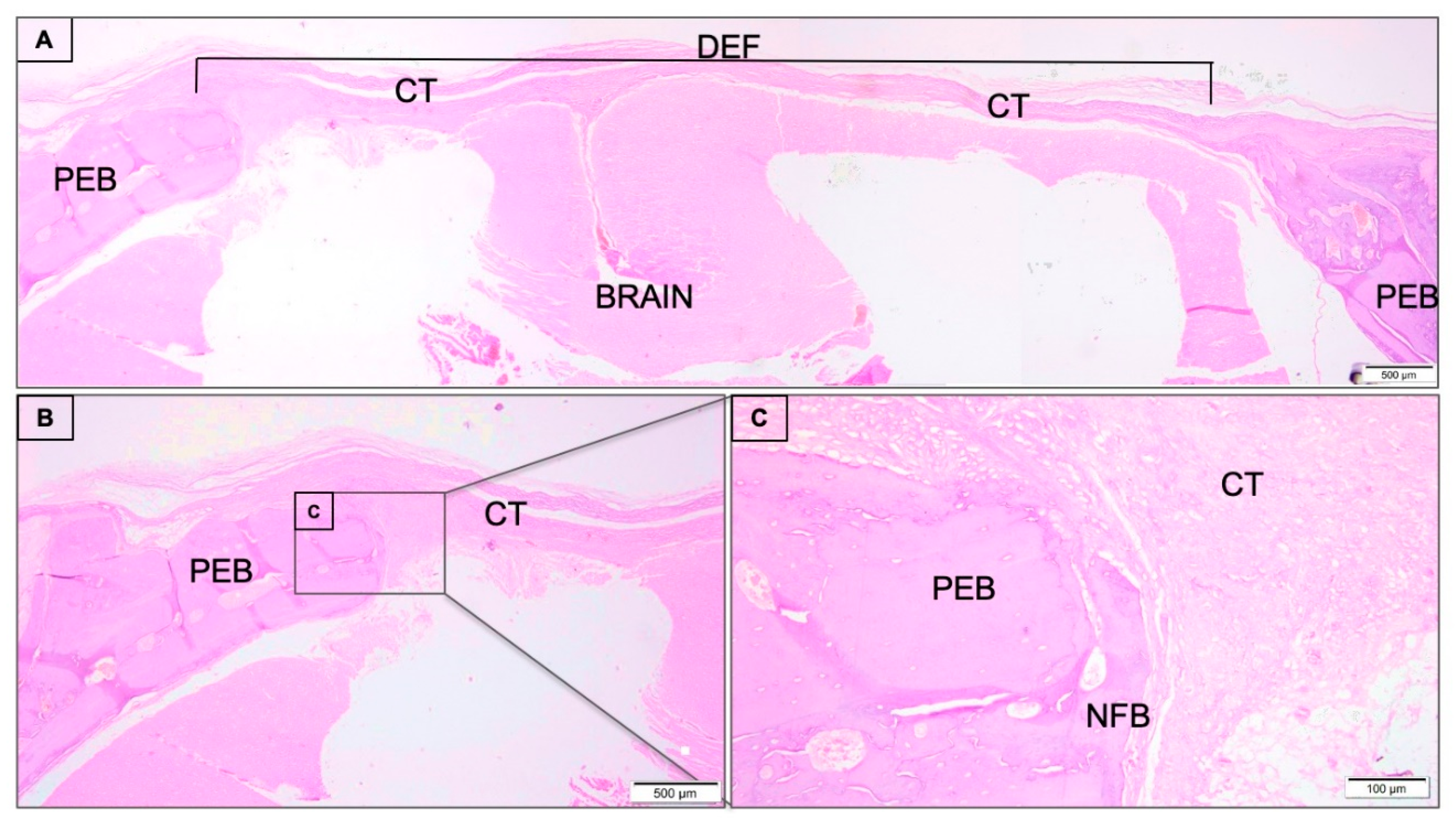
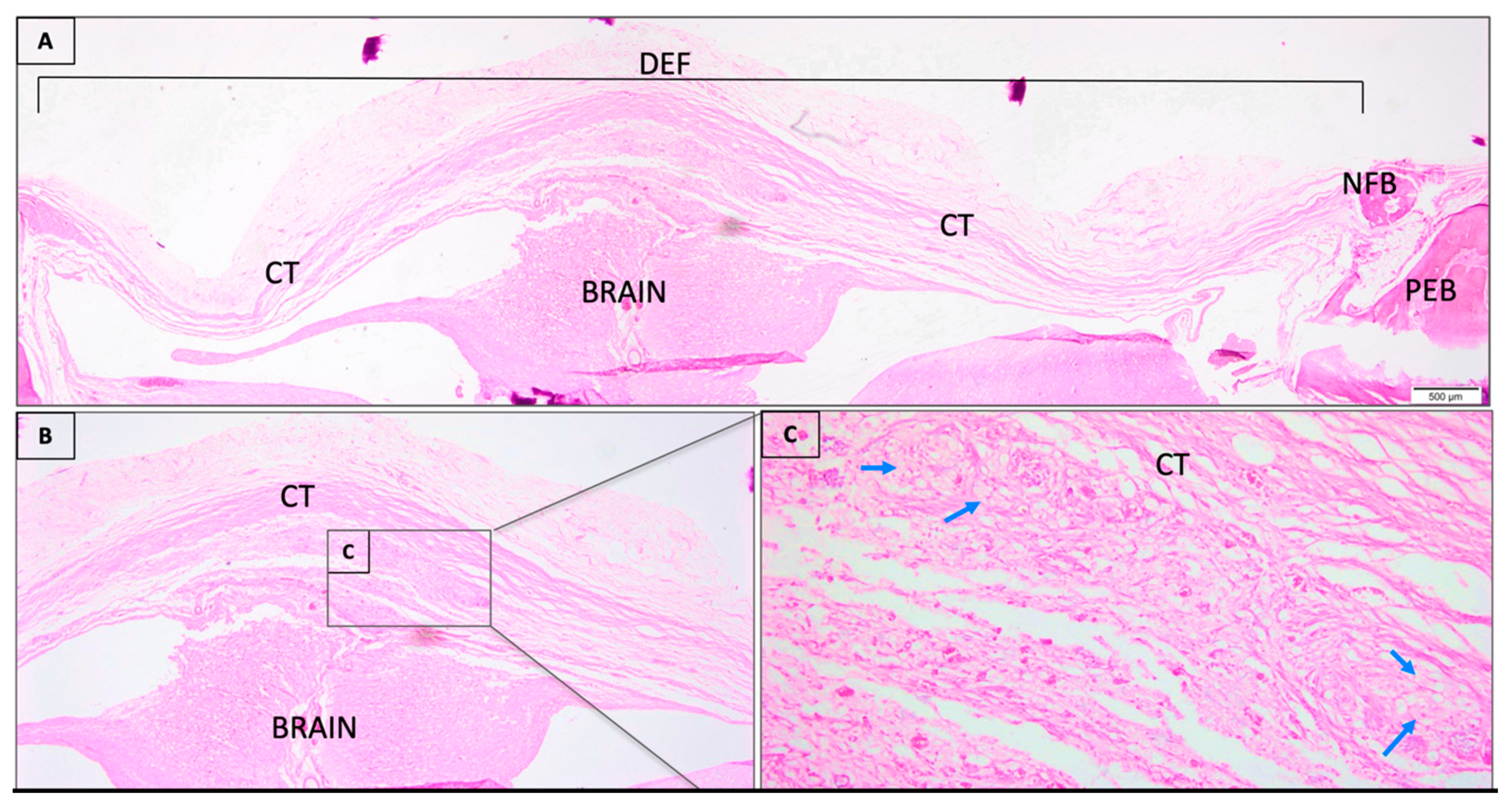
3.1. Descriptive Histological Analysis
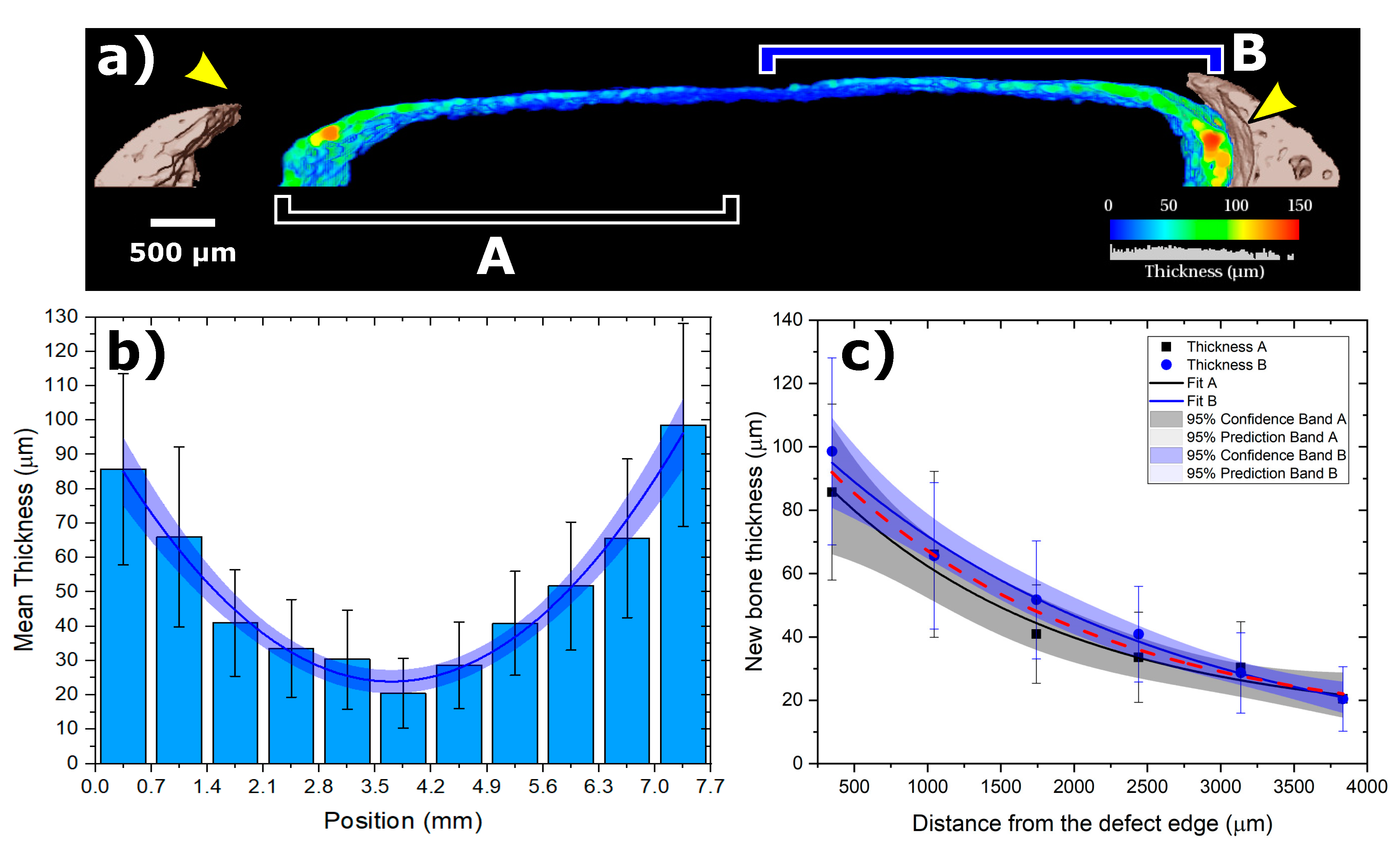
3.2. SRµCT Results
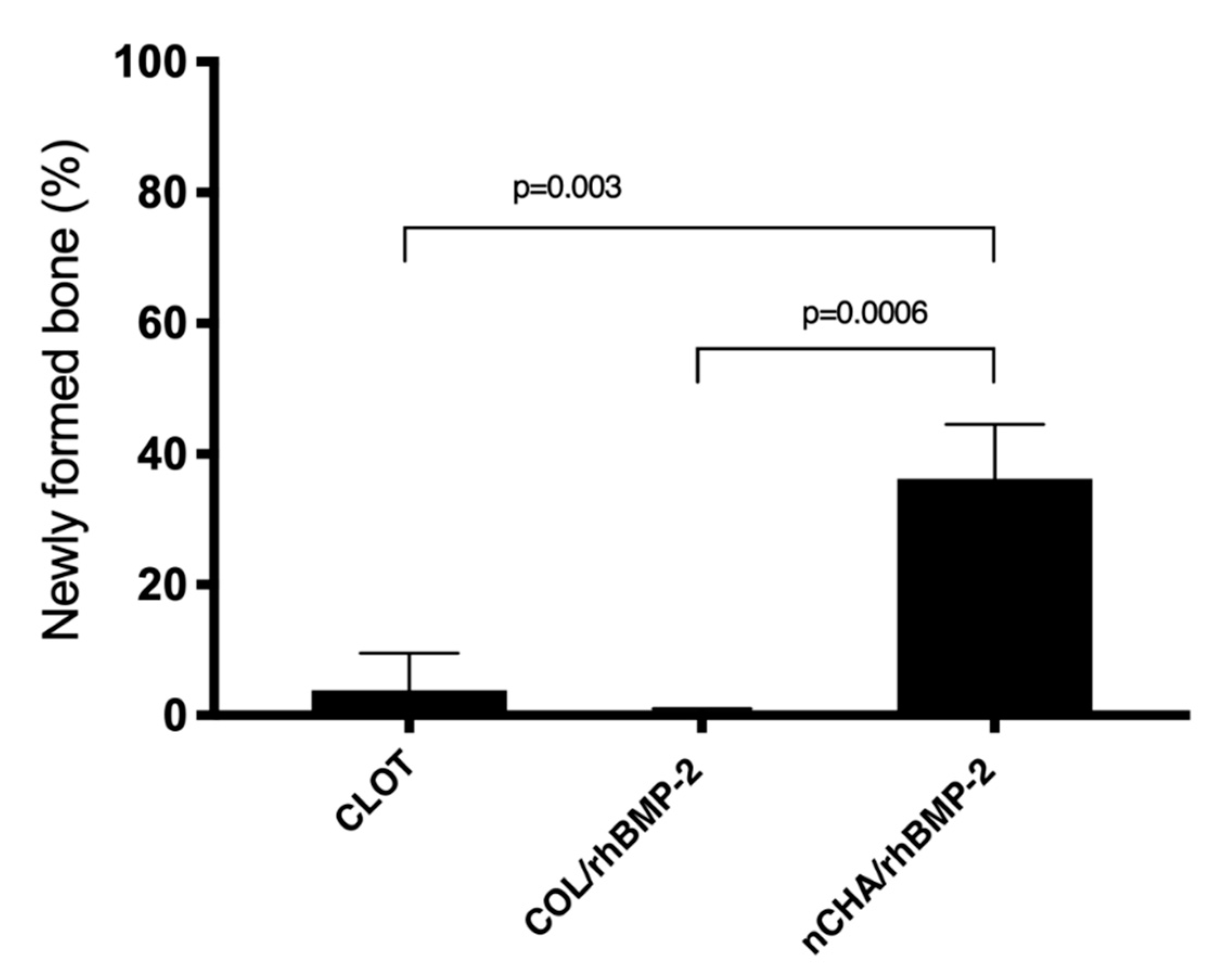
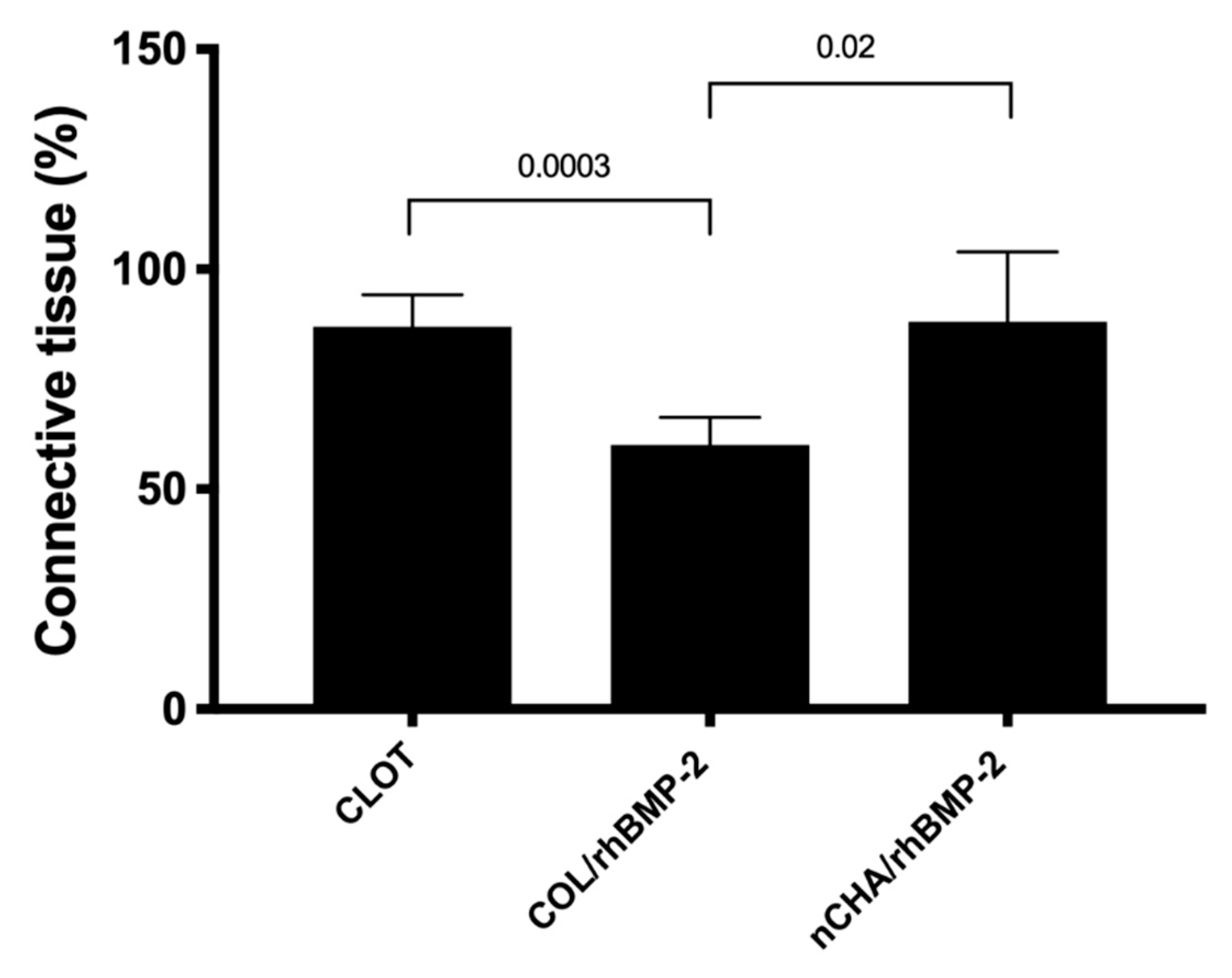
3.3. Histomorphometric Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Fernandez Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [Green Version]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.F.; Fernandes, G.V.; Santos, S.R.; Rossi, A.M.; Lima, I.; Granjeiro, J.M.; Calasans-Maia, M.D. Long-term biocompatibility evaluation of 0.5% zinc containing hydroxyapatite in rabbits. J. Mater. Sci. Mater. Med. 2013, 24, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparison of two carbonated apatite ceramics in vivo. Acta Biomater. 2010, 6, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Valiense, H.; Barreto, M.; Resende, R.F.; Alves, A.T.; Rossi, A.M.; Mavropoulos, E.; Granjeiro, J.M.; Calasans-Maia, M.D. In vitro and in vivo evaluation of strontium-containing nanostructured carbonated hydroxyapatite/sodium alginate for sinus lift in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Calasans-Maia, M.D.; Melo, B.R.; Alves, A.T.; Resende, R.F.; Louro, R.S.; Sartoretto, S.C.; Granjeiro, J.M.; Alves, G.G. Cytocompatibility and biocompatibility of nanostructured carbonated hydroxyapatite spheres for bone repair. J. Appl. Oral Sci. 2015, 23, 599–608. [Google Scholar] [CrossRef]
- Carmo, A.B.X.D.; Sartoretto, S.C.; Alves, A.T.N.N.; Granjeiro, J.M.; Miguel, F.B.; Calasans-Maia, J.; Calasans-Maia, M.D. Alveolar bone repair with strontium-containing nanostructured carbonated hydroxyapatite. J. Appl. Oral Sci. 2018, 26, e20170084. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, M.D.; Alves, A.T.N.N.; Resende, R.F.B.; da Costa Fernandes, C.J.; de Magalhães Padilha, P.; Rossi, A.M.; Teti, A.; Granjeiro, J.M.; Zambuzzi, W.F. The role of apoptosis associated speck-like protein containing a caspase-1 recruitment domain (ASC) in response to bone substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110965. [Google Scholar] [CrossRef]
- Martinez-Zelaya, V.R.; Zarranz, L.; Herrera, E.Z.; Alves, A.T.; Uzeda, M.J.; Mavropoulos, E.; Rossi, A.L.; Mello, A.; Granjeiro, J.M.; Calasans-Maia, M.D.; et al. In Vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: Zinc and calcium bioavailability and bone regeneration. Int. J. Nanomed. 2019, 14, 3471–3490. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Barros Mourão, C.F.; Lourenço, E.S.; Nascimento, J.R.B.; Machado, R.C.M.; Rossi, A.M.; Leite, P.E.C.; Granjeiro, J.M.; Alves, G.G.; Calasans-Maia, M.D. Does the association of blood-derived growth factors to nanostructured carbonated hydroxyapatite contributes to the maxillary sinus floor elevation? A randomized clinical trial. Clin. Oral Investig. 2019, 23, 369–379. [Google Scholar] [CrossRef]
- Calasans-Maia, M.D.; Barboza Junior, C.A.B.; Soriano-Souza, C.A.; Alves, A.T.N.N.; Uzeda, M.J.P.; Martinez-Zelaya, V.R.; Mavropoulos, E.; Rocha Leão, M.H.; de Santana, R.B.; Granjeiro, J.M.; et al. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019, 14, 4559–4571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, J.M.; Oliveira, R.C.; Bustos-Valenzuela, J.C.; Sogayar, M.C.; Taga, R. Bone morphogenetic proteins: From structure to clinical use. Braz. J. Med. Biol. Res. 2005, 38, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials (Basel) 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Issa, J.P.; Bentley, M.V.; Iyomasa, M.M.; Sebald, W.; de Albuquerque, R.F. Sustained release carriers used to delivery bone morphogenetic proteins in the bone healing process. Anat. Histol. Embryol. 2008, 37, 181–187. [Google Scholar] [CrossRef]
- David, L.; Feige, J.J.; Bailly, S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009, 20, 203–212. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luvizuto, E.R.; Tangl, S.; Zanoni, G.; Okamoto, T.; Sonoda, C.K.; Gruber, R.; Okamoto, R. The effect of BMP-2 on the osteoconductive properties of β-tricalcium phosphate in rat calvaria defects. Biomaterials 2011, 32, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Miqueles, G.; Martinez, G.; Guerrero Prado, P. Fast image reconstruction at a synchrotron laboratory. In Proceedings of the 2020 SIAM Conference on Parallel Processing for Scientific Computing, Seattle, WA, USA, 11–13 February 2020; pp. 24–34. [Google Scholar]
- Sartoretto, S.; Gemini-Piperni, S.; da Silva, R.A.; Calasans, M.D.; Rucci, N.; Pires Dos Santos, T.M.; Lima, I.B.C.; Rossi, A.M.; Alves, G.; Granjeiro, J.M.; et al. Apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC) contributes to osteoblast differentiation and osteogenesis. J. Cell. Physiol. 2019, 234, 4140–4153. [Google Scholar] [CrossRef]
- Gomes, P.S.; Fernandes, M.H. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef]
- Jordan, H.V. Rodent model systems in peri-odontal disease research. J. Dent. Res. 1971, 50, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Seol, Y.J.; Gruber, R.; Giannobile, W.V. Preclinical models for oral and peri-odontal reconstructive therapies. J. Dent. Res. 2009, 88, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for cranio-mandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimoes, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implants Res. 2014, 25, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Cuozzo, R.C.; Sartoretto, S.C.; Resende, R.F.B.; Alves, A.T.N.N.; Mavropoulos, E.; da Prado Silva, M.H.; Calasans-Maia, M.D. Biological evaluation of zinc-containing calcium alginate-hydroxyapatite composite microspheres for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- de MateSanchez Val, J.E.; Calvo-Guirado, J.L.; Gomez-Moreno, G.; Martınez, C.P.-A.; Mazon, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials, and SEM analysis. Clin. Oral Implants Res. 2016, 27, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.F.B.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.N.N.; Calasans-Maia, J.A.; Rossi, A.M.; Granjeiro, J.M.; Calasans-Maia, M.D. Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials (Basel) 2019, 12, 3645. [Google Scholar] [CrossRef] [Green Version]
- Coathup, M.J.; Cai, Q.; Campion, C.; Buckland, T.; Blunn, G.W. The effect of particle size on the osteointegration of injectable silicate-substituted calcium phosphate bone substitute materials. J. Biomed. Mater. Res. Part B 2013, 101, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Zelaya, V.R.; Archilha, N.L.; Calasans-Maia, M.; Farina, M.; Rossi, A.M. Trabecular architecture during the healing process of a tibial diaphysis defect. Acta Biomater. 2020, 26, S1742–S7061. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef]
- Xiao, W.; Fu, H.; Rahaman, M.N.; Liu, Y.; Bal, B.S. Hollow hydroxyapatite microspheres: A novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 2013, 9, 8374–8383. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Jeong, W.S.; Yang, S.J.; Park, E.J.; Oh, T.S.; Koh, K.S. Appropriate and Effective Dosage of BMP-2 for the Ideal Regeneration of Calvarial Bone Defects in Beagles. Plast. Reconstr. Surg. 2016, 138, 64e–72e. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider Werner Vianna, T.; Sartoretto, S.C.; Neves Novellino Alves, A.T.; Figueiredo de Brito Resende, R.; de Almeida Barros Mourão, C.F.; de Albuquerque Calasans-Maia, J.; Martinez-Zelaya, V.R.; Malta Rossi, A.; Granjeiro, J.M.; Calasans-Maia, M.D.; et al. Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria. J. Funct. Biomater. 2020, 11, 87. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040087
Schneider Werner Vianna T, Sartoretto SC, Neves Novellino Alves AT, Figueiredo de Brito Resende R, de Almeida Barros Mourão CF, de Albuquerque Calasans-Maia J, Martinez-Zelaya VR, Malta Rossi A, Granjeiro JM, Calasans-Maia MD, et al. Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria. Journal of Functional Biomaterials. 2020; 11(4):87. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040087
Chicago/Turabian StyleSchneider Werner Vianna, Thiago, Suelen Cristina Sartoretto, Adriana Terezinha Neves Novellino Alves, Rodrigo Figueiredo de Brito Resende, Carlos Fernando de Almeida Barros Mourão, Jose de Albuquerque Calasans-Maia, Victor R. Martinez-Zelaya, Alexandre Malta Rossi, Jose Mauro Granjeiro, Monica Diuana Calasans-Maia, and et al. 2020. "Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria" Journal of Functional Biomaterials 11, no. 4: 87. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb11040087





