Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole
Abstract
:1. Introduction
Why CR232-G5K NPs and Not a Liposome-Based Formulation?
2. Materials and Methods
2.1. Chemical Substances and Instruments
2.2. Microbiology
2.2.1. Bacterial Species Considered in This Study
2.2.2. Determination of the Minimal Inhibitory Concentrations (MICs)
2.2.3. Time-Kill Experiments
2.3. Evaluation of Cytotoxicity of CR232, G5K, and CR232-G5K NPs on Human Keratinocytes
2.3.1. Experimental Protocol for Cell Culture
2.3.2. Viability Assay
2.4. Statistical Analyses
3. Results and Discussion
3.1. Brief Recapitulation of the Main Characteristics of CR232-G5K NPs
3.2. Antibacterial Effects of CR232-G5K NPs
3.2.1. Determination of MIC Values
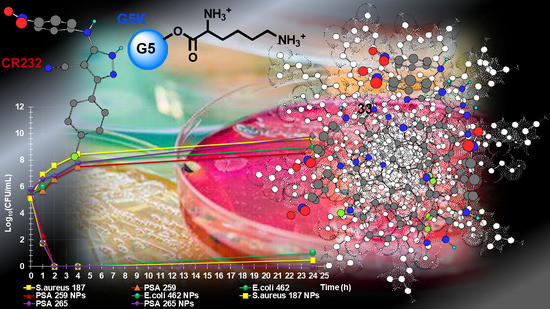
3.2.2. Time-Killing Curves
3.3. Cytotoxicity of G5K, CR232, and CR232-G5K NPs on HaCaT Human Keratinocytes Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Interagency Coordination Group on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 15 February 2022).
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songmee, B.; Jaehoon, L.; Jaehwa, L.; Eunah, K.; Sunhwa, L.; Jaeyon, Y.; Yeonho, K. Antimicrobial Resistance in Haemophilus influenzae Respiratory Tract Isolates in Korea: Results of a Nationwide Acute Respiratory Infections Surveillance. Antimicrob. Agents Chemother. 2010, 54, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfei, S.; Brullo, C.; Caviglia, D.; Piatti, G.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Schito, A.M. Pyrazole-Based Water-Soluble Dendrimer Nanoparticles as a Potential New Agent against Staphylococci. Biomedicines 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P. Antibiotic resistance¸causes and consequences. Eur. J. Biomed. Pharm. Sci. 2020, 7, 327–331. [Google Scholar]
- World Health Organization (WHO). Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 15 February 2022).
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Available online: https://www.paho.org/en/topics/antimicrobial-resistance (accessed on 5 April 2022).
- Asenjo, A.; Oteo-Iglesias, J.; Alós, J.I. What’s new in mechanisms of antibiotic resistance in bacteria of clinical origin? Enferm. Infecc. Microbiol. Clin. 2021, 39, 291–299. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, J.; Wang, Y.; Verstraete, W.; Yuan, Z.; Guo, J. Insights of Metallic Nanoparticles and Ions in Accelerating the Bacterial Uptake of Antibiotic Resistance Genes. J. Hazard. Mater. 2022, 421, 126728. [Google Scholar] [CrossRef]
- PBS New Hour. We’re Headed towards a ‘Post-Antibiotic Era’, World Health Organization Warns. Available online: https://www.pbs.org/newshour/health/world-health-organization-warns-headed-post-antibiotic-era#:~:text=%E2%80%9CWithout%20urgent%2C%20coordinated%20action%20by%20many%20stakeholders%2C%20the,Keiji%20Fukuda%2C%20WHO%E2%80%99s%20Assistant%20Director-General%20for%20Health%20Security (accessed on 15 February 2022).
- UN News. Global Perspective Human Stories. Available online: https://news.un.org/en/story/2016/11/545322-without-urgent-action-world-heading-towards-post-antibiotic-era-un-health (accessed on 15 February 2022).
- United Nations; Chang, Y.; Yao, Y.; Cui, Z.; Yang, G.; Li, D.; Wang, L.; Tang, L. Changing antibiotic prescribing practices in outpatient primary care settings in China: Study protocol for a health information system-based cluster-randomised crossover controlled trial. PLoS ONE 2022, 17, e0259065. [Google Scholar] [CrossRef]
- EMA Categorisation of Antibiotics for Use in Animals for Prudent and Responsible Use. Available online: https://www.ema.europa.eu/en/documents/report/infographic-categorisation-antibiotics-use-animals-prudent-responsible-use_en.pdf (accessed on 15 February 2022).
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Bactericidal Activity of Non-Cytotoxic Cationic Nanoparticles against Clinically and Environmentally Relevant Pseudomonas spp. Isolates. Pharmaceutics 2021, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. Antibacterial Pyrazoles: Tackling Resistant Bacteria. Future Med. Chem. 2022, 14, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.; Rotolo, C.; Ponassi, M.; Iervasi, E.; Rosano, C.; Spallarossa, A. One-pot synthesis and antiproliferative activity of highly functionalized pyrazole derivatives. ChemMedChem 2022, 17, e202100670. [Google Scholar] [CrossRef]
- Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Alfei, S. Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride. Int. J. Mol. Sci. 2021, 22, 5021. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria/ (accessed on 19 March 2022).
- Schito, A.M.; Piatti, G.; Stauder, M.; Bisio, A.; Giacomelli, E.; Romussi, G.; Pruzzo, C. Effects of demethylfruticuline A and fruticuline A from Salvia corrugata Vahl. on biofilm production in vitro by multiresistant strains of Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis. Int. J. Antimicrob. Agents 2011, 37, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, D.; Pastorino, F.; Zuccari, G.; Caffa, I.; Loi, M.; Marimpietri, D.; Brignole, C.; Perri, P.; Cilli, M.; Nico, B.; et al. Enhanced Anti-Tumor and Anti-Angiogenic Efficacy of a Novel Liposomal Fenretinide on Human Neuroblastoma. J. Control. Release 2013, 170, 445–451. [Google Scholar] [CrossRef]
- Nada, A.; Al-Moghazy, M.; Soliman, A.A.F.; Rashwan, G.M.T.; Eldawy, T.H.A.; Hassan, A.A.E.; Sayed, G.H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production. Pharmaceuticals 2022, 15, 384. [Google Scholar] [CrossRef]
- Alfei, S.; Brullo, C.; Caviglia, D.; Zuccari, G. Preparation and Physicochemical Characterization of Water-Soluble Pyrazole-Based Nanoparticles by Dendrimer Encapsulation of an Insoluble Bioactive Pyrazole Derivative. Nanomaterials 2021, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, L.; Gao, M.; Que, X.; Zhou, C.; Zhu, D.; Cai, Y. Nanoformulation of a Novel Pyrano[2,3-c] Pyrazole Heterocyclic Compound AMDPC Exhibits Anti-Cancer Activity via Blocking the Cell Cycle through a P53-Independent Pathway. Molecules 2019, 24, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimizadeh, M.; Pordel, M.; Bakavoli, M.; Rezaeian, S.; Sadeghian, A. Synthesis and Antibacterial Activity of Some New Derivatives of Pyrazole. World J. Microbiol. Biotechnol. 2010, 26, 317–321. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Abdel-Aziem, T. Design, Synthesis and Biological Evaluation of Some Pyrazole Derivatives as Anti-Inflammatory-Antimicrobial Agents. Bioorg. Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Shetty, P.; Sundershan, S.; Fun, H.K. New Pyrazole Derivatives Containing 1,2,4-Triazoles and Benzoxazoles as Potent Antimicrobial and Analgesic Agents. Eur. J. Med. Chem. 2013, 62, 410–415. [Google Scholar] [CrossRef]
- Ebenezer, O.; Singh-Pillay, A.; Koorbanally, N.A.; Singh, P. Antibacterial Evaluation and Molecular Docking Studies of Pyrazole–Thiosemicarbazones and Their Pyrazole–Thiazolidinone Conjugates. Mol. Divers. 2021, 25, 191–204. [Google Scholar] [CrossRef]
- Ahmed, W.; Yan, X.; Hu, D.; Adnan, M.; Tang, R.; Cui, Z.-N. Synthesis and fungicidal activity of novel pyrazole derivatives containing 5-Phenyl-2-Furan. Bioorg. Med. Chem. 2019, 27, 115048. [Google Scholar] [CrossRef]
- Ma, H.-J.; Li, Y.-H.; Zhao, Q.-F.; Zhang, T.; Xie, R.-L.; Mei, X.-D.; Ning, J. Synthesis and Herbicidal Activity of Novel N-(2,2,2)-Trifluoroethylpyrazole Derivatives. J. Agric. Food Chem. 2010, 58, 4356–4360. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.-Q.; Liu, J.; Yang, X.-P.; Liu, Z.-J. Stereoselective Synthesis and Antifungal Activities of (E)-α-(Methoxyimino) Benzeneacetate Derivatives Containing 1,3,5-Substituted Pyrazole Ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef]
- Adamu, M.; Naidoo, V.; Eloff, J.N. The Antibacterial Activity, Antioxidant Activity and Selectivity Index of Leaf Extracts of Thirteen South African Tree Species Used in Ethnoveterinary Medicine to Treat Helminth Infections. BMC Vet. Res. 2014, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Adamu, M.; Naidoo, V.; Eloff, J.N. Efficacy and Toxicity of Thirteen Plant Leaf Acetone Extracts Used in Ethnoveterinary Medicine in South Africa on Egg Hatching and Larval Development of Haemonchus Contortus. BMC Vet. Res. 2013, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Acetone Leaf Extracts of Nine Under-Investigated South African Eugenia and Syzygium (Myrtaceae) Species and Their Selectivity Indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, F.; do Rosario, V.E. Methods for assessment of antimalarial activity in the different phases of the Plasmodiumlife cycle. Rev. Pan Amaz. Saude 2010, 1, 109–1245. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Resveratrol-loaded D-tocopheryl polyethylene glycol 1000 succinate micelles as nutritional supplement for children with chronic liver disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Considerable Improvement of Ursolic Acid Water Solubility by Its Encapsulation in Dendrimer Nanoparticles: Design, Synthesis and Physicochemical Characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef]
- Benns, J.M.; Choi, J.S.; Mahato, R.I.; Park, J.S.; Kim, S.W. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(l-histidine)-graft-poly(l-lysine) comb shaped polymer. Bioconj. Chem. 2000, 11, 637–645. [Google Scholar] [CrossRef]




| Analysis | CR232-G5K NPs | |
|---|---|---|
| FTIR [cm−1] |  G5K (green line), CR232 (black line), CR232-G5K NPs (red line) | |
| 1H NMR (400 MHz, DMSO-d6) [ppm] |  | |
| 13C NMR (100 MHz, DMSO-d6) [ppm] |  | |
| UV-Vis | Ultraviolet Spectrum | ʎ abs = 328 nm |
| UV-Vis | DL (%) | 31.7 ± 0.6 |
| EE (%) | 98.3 ± 2.0 | |
| 1H NMR | MW | 44153.1 44219.5 ± 237.8 |
| DL% (UV-Vis) | ||
| Scanning Electron Microscopy (SEM) | Morphology | Spherical |
| Average Size | ≃500 nm | |
| DLS 1 Analysis | Z-Ave 2 (nm) PDI 3 | 529.7 ± 33.5 5 0.427 ± 0.054 5 |
| ζ-p 4 (mV) | +37.2 ± 7.0 5 | |
| Solubilization Essay | Water-Solubility (mg/mL) | 5.2 ±0.05 6,§,8 1.65 ± 0.02 7,§,9 |
| Dialysis Method (UV-Vis) | Cumulative Release (%, 24 h) | 99.3 |
| Mathematical Model | Weibull (β > 1) | |
| Mechanism | Complex Mechanisms | |
| Cytotoxicity of G5K (HeLa Cells) | LD50 | 64.4 µM * |
| Potentiometric Titration | Buffer Capacity (β) | 0.3076 0.1871 |
| Average Buffer Capacity (β mean) | ||
| CR232-G5K NPs (44,220) 2 | CR232 (339.7) 2 | ||||
|---|---|---|---|---|---|
| Strains | MIC µM (µg/mL) | MBC µM (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | Selectivity Indices 3 |
| Enterococcus genus | |||||
| E. faecalis 1 * | 0.72 (32) | 1.44 (64) | 10.1 20.2 10.1 | 20.2 20.2 20.2 | 8 |
| E. faecalis 365 * | 1.44 (64) | 1.44 (64) | 4 | ||
| E. faecalis 439 * | 0.72 (32) | 1.44 (64) | 8 | ||
| E. faecium 21 | 0.36 (16) | 0.72 (32) | 5.05 5.05 5.05 | 10.1 10.1 10.1 | 16 |
| E. faecium 300 * | 0.36 (16) | 0.72 (32) | 16 | ||
| E. faecium 369 * | 0.36 (16) | 0.72 (32) | 16 | ||
| Staphylococcus genus | |||||
| S. aureus 18 ** | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| S. aureus 187 ** | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| S. aureus 188 ** | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| S. aureus 189 ** | 1.44 (64) | 1.44 (64) | 20.2 | 20.2 | 4 |
| S. epidermidis 22 ** | 0.36 (16) | 0.72 (32) | 5.05 | 10.1 | 16 |
| S. epidermidis 178 | 0.36 (16) | 0.72 (32) | 5.05 | 10.1 | 16 |
| S. epidermidis 181 *** | 0.36 (16) | 0.72 (32) | 5.05 | 10.1 | 16 |
| Sporogenic isolate | |||||
| B. subtilis | 0.36 (16) | 0.36 (16) | 5.05 | 5.05 | 16 |
| CR232-G5K NPs (44,220) 2 | CR232 (339.7) 2 | ||||
|---|---|---|---|---|---|
| Strains | MIC µM (µg/mL) | MBC µM (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | Selectivity Indices 3 |
| Enterobacteriaceae family | |||||
| E. coli 224 S | 0.72 (32) | 0.72 (32) | 10.1 | 10.1 | 8 |
| E. coli 376# | 1.44 (64) | 1.44 (64) | 20.2 | 20.2 | 4 |
| E. coli 462 § | 0.72 (32) | 0.72 (32) | 10.1 | 10.1 | 8 |
| P. mirabilis 254 | >2.89 (128) | N.D. | >40.4 | N.D. | <2 |
| M. morganii 372 | >2.89 (128) | N.D. | >40.4 | N.D. | <2 |
| K. pneumoniae 375# | 1.44 (64) | 2.89 (128) | 20.2 | 40.4 | 4 |
| K. pneumoniae 376# | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| K. pneumoniae 377# | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| K. pneumoniae 490#CR | 0.72 (32) | 2.89 (128) | 20.2 | 40.4 | 8 |
| Salmonella gr. B 227 | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| P. stuartii 374 | >2.89 (128) | N.D. | >40.4 | N.D. | <2 |
| Non-fermenting species | |||||
| A. baumannii 257 | 1.44 (64) | 1.44 (64) | 20.2 | 20.2 | 4 |
| A. baumannii 279 | 0.72 (32) | 0.72 (32) | 10.1 | 10.1 | 8 |
| A. baumannii 245 | 1.44 (64) | 1.44 (64) | 20.2 | 20.2 | 4 |
| P. aeruginosa 1 V | 0.72 (32) | 1.44 (64) | 10.1 | 20.2 | 8 |
| P. aeruginosa 265 CR | 0.72 (32) | 1.44 (64) | 10.1 | 20.2 | 8 |
| P. aeruginosa 432 | 0.72 (32) | 0.72 (32) | 10.1 | 10.1 | 8 |
| P. aeruginosa 259 * | 2.89 (128) | 2.89 (128) | 40.4 | 40.4 | 2 |
| S. maltophilia 466 | 0.72 (32) | 1.44 (64) | 10.1 | 20.2 | 8 |
| S. maltophilia 390 | 0.72 (32) | 1.44 (64) | 10.1 | 20.2 | 8 |
| S. maltophilia 392 | 1.44 (64) | 2.89 (128) | 20.2 | 40.4 | 4 |
| S. maltophilia 392 | 0.72 (32) | 0.72 (32) | 10.1 | 10.1 | 8 |
| Strains | CR232-G5K NPs (44,220) 2 | CR232 Released (339.7) 2 | Reference Antibiotics |
|---|---|---|---|
| MIC µM (µg/mL) | MIC µM (µg/mL) | MIC µM (µg/mL) | |
| E. faecalis 1 * | 0.72 (32) | 29.7 (10.1) | 193.2 (64) 3 193.2 (64) 3 193.2 (64) 3 |
| E. faecalis 365 * | 1.44 (64) | 59.5 (20.2) | |
| E. faecalis 439 * | 0.72 (32) | 29.7 (10.1) | |
| E. faecium 21 | 0.36 (16) | 14.9 (5.05) | 700.6 (256) 3 700.6 (256) 3 700.6 (256) 3 |
| E. faecium 300 * | 0.36 (16) | 14.9 (5.05) | |
| E. faecium 369 * | 0.36 (16) | 14.9 (5.05) | |
| S. aureus 18 ** | 2.89 (128) | 119.0 (40.4) | 386.4 (128) 3, 1401.2 (512) 4 |
| S. aureus 187 ** | 2.89 (128) | 119.0 (40.4) | 386.4 (128) 3, 1401.2 (512) 4 |
| S. aureus 188 ** | 2.89 (128) | 119.0 (40.4) | 386.4 (128) 3, 1401.2 (512) 4 |
| S. aureus 189 ** | 1.44 (64) | 59.5 (20.2) | 386.4 (128) 3, 1401.2 (512) 4 |
| S. epidermidis 22 ** | 0.36 (16) | 14.9 (5.05) | 193.2 (64) 3, 700.6 (256) 4 |
| S. epidermidis 178 | 0.36 (16) | 14.9 (5.05) | 193.2 (64) 3, 700.6 (256) 4 |
| S. epidermidis 181 *** | 0.36 (16) | 14.9 (5.05) | 193.2 (64) 3, 700.6 (256) 4 |
| B. subtilis | 0.36 (16) | 14.9 (5.05) | 212.4 (128) 5 |
| Strains | CR232-G5K NP (44,220) 2 | CR232 Released (339.7) 2 | Reference Antibiotics |
|---|---|---|---|
| MIC µM (µg/mL) | MIC µM (µg/mL) | MIC µM (µg/mL) | |
| E. coli 224 S | 1.44 (64) | 59.5 (20.2) | 96.6 (32) 3 |
| E. coli 376# | 1.44 (64) | 59.5 (20.2) | 96.6 (32) 3 |
| E. coli 462§ | 1.44 (64) | 59.5 (20.2) | 96.6 (32) 3 |
| K. pneumoniae 375# | 1.44 (64) | 59.5 (20.2) | 96.6 (32) 3 |
| K. pneumoniae 376# | 2.89 (128) | 119.0 (40.4) | 96.6 (32) 3 |
| K. pneumoniae 377# | 2.89 (128) | 119.0 (40.4) | 96.6 (32) 3 |
| K. pneumoniae 490#CR | 0.72 (32) | 29.7 (10.1) | 18.5 (16) 4 |
| Salmonella gr. B 227 | 2.89 (128) | 119.0 (40.4) | 235.5 (128) 5 |
| A. bawmannii 257 | 1.44 (64) | 59.5 (20.2) | 193.2 (64) 3 |
| A. bawmannii 279 | 0.72 (32) | 29.7 (10.1) | 193.2 (64) 3 |
| A. bawmannii 245 | 1.44 (64) | 59.5 (20.2) | 193.2 (64) 3 |
| P. aeruginosa 1 V | 0.72 (32) | 29.7 (10.1) | 76.2 (64) 6 |
| P. aeruginosa 265 CR | 0.72 (32) | 29.7 (10.1) | 18.5 (16) 4 |
| P. aeruginosa 432 | 0.72 (32) | 29.7 (10.1) | 76.2 (64) 6 |
| P. aeruginosa 259 * | 2.89 (128) | 119.0 (40.4) | 76.2 (64) 6 |
| S. maltophilia 466 | 0.72 (32) | 29.7 (10.1) | 117.7 (64) 5 |
| S. maltophilia 390 | 0.72 (32) | 29.7 (10.1) | 117.7 (64) 5 |
| S. maltophilia 392 | 1.44 (64) | 59.5 (20.2) | 117.7 (64) 5 |
| S. maltophilia 392 | 0.72 (32) | 29.7 (10.1) | 117.7 (64) 5 |
| Sample | Equations | R2 | LD50 (µg/mL;µM) | SI |
|---|---|---|---|---|
| G5K | y = 0.0146x2 − 2.2620x + 87.8850 | 0.9625 | 577.40; 19.10 | N.A. |
| CR232 | y = 0.0146x2 − 2.2968x + 93.1770 | 0.9292 | 7.41; 21.83 | ≤0.05789 |
| CR232-G5K NPs | y = 90.613e−0.126x | 0.9474 | 247.6; 5.6 | 2–16 1 |
| CR232 provided by NPs | y = 92.160e−0.003x | 0.9618 | 80.2; 236.1 | 2–16 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Caviglia, D.; Zorzoli, A.; Marimpietri, D.; Spallarossa, A.; Lusardi, M.; Zuccari, G.; Schito, A.M. Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole. Biomedicines 2022, 10, 907. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040907
Alfei S, Caviglia D, Zorzoli A, Marimpietri D, Spallarossa A, Lusardi M, Zuccari G, Schito AM. Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole. Biomedicines. 2022; 10(4):907. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040907
Chicago/Turabian StyleAlfei, Silvana, Debora Caviglia, Alessia Zorzoli, Danilo Marimpietri, Andrea Spallarossa, Matteo Lusardi, Guendalina Zuccari, and Anna Maria Schito. 2022. "Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole" Biomedicines 10, no. 4: 907. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10040907