Efficacy of Olanzapine for High and Moderate Emetogenic Chemotherapy in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Chemotherapy Emetogenicity Assessment
2.3. Standard CIV Prophylaxis
2.4. Olanzapine Treatment
2.5. Response Assessment
2.6. Ethical Approval
3. Results
3.1. Patient Characteristics
3.2. Olanzapine Usage
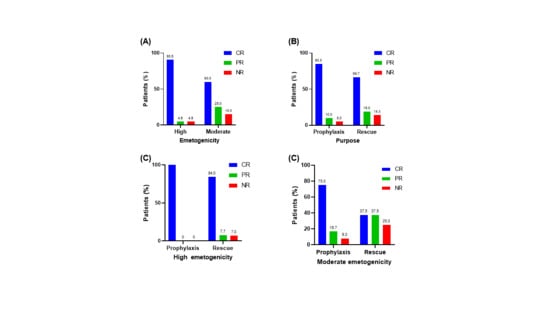
3.3. Efficacy of Olanzapine
3.4. Adverse Events during Olanzapine Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, H.J.; Loftus, S.; Taylor, A.; DiCristina, C.; Green, S.; Zwaan, C.M. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in children: A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 385–394. [Google Scholar] [CrossRef]
- Flank, J.; Schechter, T.; Gibson, P.; Johnston, D.L.; Orsey, A.D.; Portwine, C.; Sung, L.; Dupuis, L.L. Olanzapine for prevention of chemotherapy-induced nausea and vomiting in children and adolescents: A multi-center, feasibility study. Support. Care Cancer 2018, 26, 549–555. [Google Scholar] [CrossRef]
- Flank, J.; Thackray, J.; Nielson, D.; August, A.; Schechter, T.; Alexander, S.; Sung, L.; Dupuis, L.L. Olanzapine for treatment and prevention of acute chemotherapy-induced vomiting in children: A retrospective, multi-center review. Pediatr. Blood Cancer 2015, 62, 496–501. [Google Scholar] [CrossRef]
- Tanaka, K.; Inui, N.; Karayama, M.; Yasui, H.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; Nakamura, Y.; et al. Olanzapine-containing antiemetic therapy for the prevention of carboplatin-induced nausea and vomiting. Cancer Chemother. Pharmacol. 2019, 84, 147–153. [Google Scholar] [CrossRef]
- Krause, M.; Zhu, Y.; Huhn, M.; Schneider-Thoma, J.; Bighelli, I.; Chaimani, A.; Leucht, S. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: A network meta-analysis. Eur. Neuropsychopharmacol. 2018, 28, 659–674. [Google Scholar] [CrossRef]
- Pagsberg, A.K.; Tarp, S.; Glintborg, D.; Stenstrom, A.D.; Fink-Jensen, A.; Correll, C.U.; Christensen, R. Acute Antipsychotic Treatment of Children and Adolescents With Schizophrenia-Spectrum Disorders: A Systematic Review and Network Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 191–202. [Google Scholar] [CrossRef]
- Strawn, J.R.; Delbello, M.P. Olanzapine for the treatment of bipolar disorder in children and adolescents. Expert Opin. Pharmacother. 2008, 9, 467–474. [Google Scholar] [CrossRef]
- Flank, J.; Sung, L.; Dvorak, C.C.; Spettigue, W.; Dupuis, L.L. The safety of olanzapine in young children: A systematic review and meta-analysis. Drug Saf. 2014, 37, 791–804. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Han, H.S.; Bae, W.K.; Park, M.R.; Shim, H.; Lee, S.C.; Go, S.I.; Yun, H.J.; Im, Y.J.; Song, E.K. A Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients Receiving Moderately Emetogenic Chemotherapy: Results of the Korean South West Oncology Group (KSWOG) Study. Cancer Res. Treat. 2019, 51, 90–97. [Google Scholar] [CrossRef]
- Dulal, S.; Paudel, B.D.; Neupane, P.; Shah, A.; Acharya, B.; Poudyal, B.S.; Shilpakar, R.; Wood, L.A. Randomized Phase II Trial to Compare the Efficacy of Haloperidol and Olanzapine in the Control of Chemotherapy-Induced Nausea and Vomiting in Nepal. J. Glob. Oncol. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Mehra, N.; Ganesan, P.; Ganesan, T.S.; Veeriah, S.; Boopathy, A.; Radhakrishnan, V.; Dhanushkodi, M.; Rajaraman, S.; Ganesharajah, S.; Sagar, T.G. Effectiveness of olanzapine in patients who fail therapy with aprepitant while receiving highly emetogenic chemotherapy. Med. Oncol. 2017, 35, 12. [Google Scholar] [CrossRef]
- Navari, R.M.; Nagy, C.K.; Gray, S.E. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support. Care Cancer 2013, 21, 1655–1663. [Google Scholar] [CrossRef]
- Navari, R.M.; Einhorn, L.H.; Loehrer, P.J., Sr.; Passik, S.D.; Vinson, J.; McClean, J.; Chowhan, N.; Hanna, N.H.; Johnson, C.S. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier oncology group study. Support. Care Cancer 2007, 15, 1285. [Google Scholar] [CrossRef]
- Navari, R.M.; Einhorn, L.H.; Passik, S.D.; Loehrer, P.J., Sr.; Johnson, C.; Mayer, M.L.; McClean, J.; Vinson, J.; Pletcher, W. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier Oncology Group study. Support. Care Cancer 2005, 13, 529–534. [Google Scholar] [CrossRef]
- Navari, R.M.; Qin, R.; Ruddy, K.J.; Liu, H.; Powell, S.F.; Bajaj, M.; Dietrich, L.; Biggs, D.; Lafky, J.M.; Loprinzi, C.L. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 375, 134–142. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Pai, V.; Rajaraman, S.; Mehra, N.; Ganesan, T.; Dhanushkodi, M.; Perumal Kalaiyarasi, J.; Rajan, A.K.; Selvarajan, G.; Ranganathan, R.; et al. Olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced vomiting in children: An open-label, randomized phase 3 trial. Pediatr. Blood Cancer 2020, 67, e28532. [Google Scholar] [CrossRef]
- Paw Cho Sing, E.; Robinson, P.D.; Flank, J.; Holdsworth, M.; Thackray, J.; Freedman, J.; Gibson, P.; Orsey, A.D.; Patel, P.; Phillips, R.; et al. Classification of the acute emetogenicity of chemotherapy in pediatric patients: A clinical practice guideline. Pediatr. Blood Cancer 2019, 66, e27646. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, L.L.; Boodhan, S.; Sung, L.; Portwine, C.; Hain, R.; McCarthy, P.; Holdsworth, M.; Pediatric Oncology Group of Ontario. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr. Blood Cancer 2011, 57, 191–198. [Google Scholar] [CrossRef]
- Dupuis, L.L.; Boodhan, S.; Holdsworth, M.; Robinson, P.D.; Hain, R.; Portwine, C.; O′Shaughnessy, E.; Sung, L.; Pediatric Oncology Group of Ontario. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr. Blood Cancer 2013, 60, 1073–1082. [Google Scholar] [CrossRef]
- Zanglis, A.; Valsamaki, P.; Fountos, G. Erdheim-Chester disease: Symmetric uptake in the (99m)Tc-MDP bone scan. Hell. J. Nucl. Med. 2008, 11, 164–167. [Google Scholar]
- Navari, R.M.; Gray, S.E.; Kerr, A.C. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A randomized phase III trial. J. Support Oncol. 2011, 9, 188–195. [Google Scholar] [CrossRef]

| Characteristics | N (%) | |
|---|---|---|
| Age | Mean ± SD | 13.2 ± 4.8 |
| Median (range) | 15.1 (4.0–18.0) | |
| Sex | Male | 12 (92.3) |
| Female | 1 (7.7) | |
| Diagnosis | Ewing’s sarcoma | 3 (23.1) |
| Acute lymphoblastic leukemia | 2 (15.4) | |
| Acute myeloid leukemia | 2 (15.4) | |
| Langerhans cell histiocytosis | 1 (7.7) | |
| Neuroblastoma | 1 (7.7) | |
| Synovial sarcoma | 1 (7.7) | |
| Osteosarcoma | 1 (7.7) | |
| Non-Hodgkin lymphoma | 1 (7.7) |
| Characteristic | N (%) | |
|---|---|---|
| Chemotherapy emetogenicity | High | 21 (51.2) |
| Moderate | 20 (48.8) | |
| Reason for olanzapine use | Prophylaxis | 20 (48.8) |
| Rescue | 21 (51.2) | |
| Chemotherapy block (days) | 4 ± 2 | |
| Duration of olanzapine use (days) | 3 ± 2 | |
| Olanzapine dose | mg/kg/dose | 0.07 ± 0.04 |
| mg/m2/dose | 2.50 ± 1.37 | |
| Olanzapine frequency | Once daily | 34 (82.9) |
| Twice daily | 7 (17.1) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.R.; Kim, S.M.; Oh, M.Y.; Lee, J.M. Efficacy of Olanzapine for High and Moderate Emetogenic Chemotherapy in Children. Children 2020, 7, 140. https://0-doi-org.brum.beds.ac.uk/10.3390/children7090140
Lee SR, Kim SM, Oh MY, Lee JM. Efficacy of Olanzapine for High and Moderate Emetogenic Chemotherapy in Children. Children. 2020; 7(9):140. https://0-doi-org.brum.beds.ac.uk/10.3390/children7090140
Chicago/Turabian StyleLee, So Rae, Su Min Kim, Min Young Oh, and Jae Min Lee. 2020. "Efficacy of Olanzapine for High and Moderate Emetogenic Chemotherapy in Children" Children 7, no. 9: 140. https://0-doi-org.brum.beds.ac.uk/10.3390/children7090140