Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
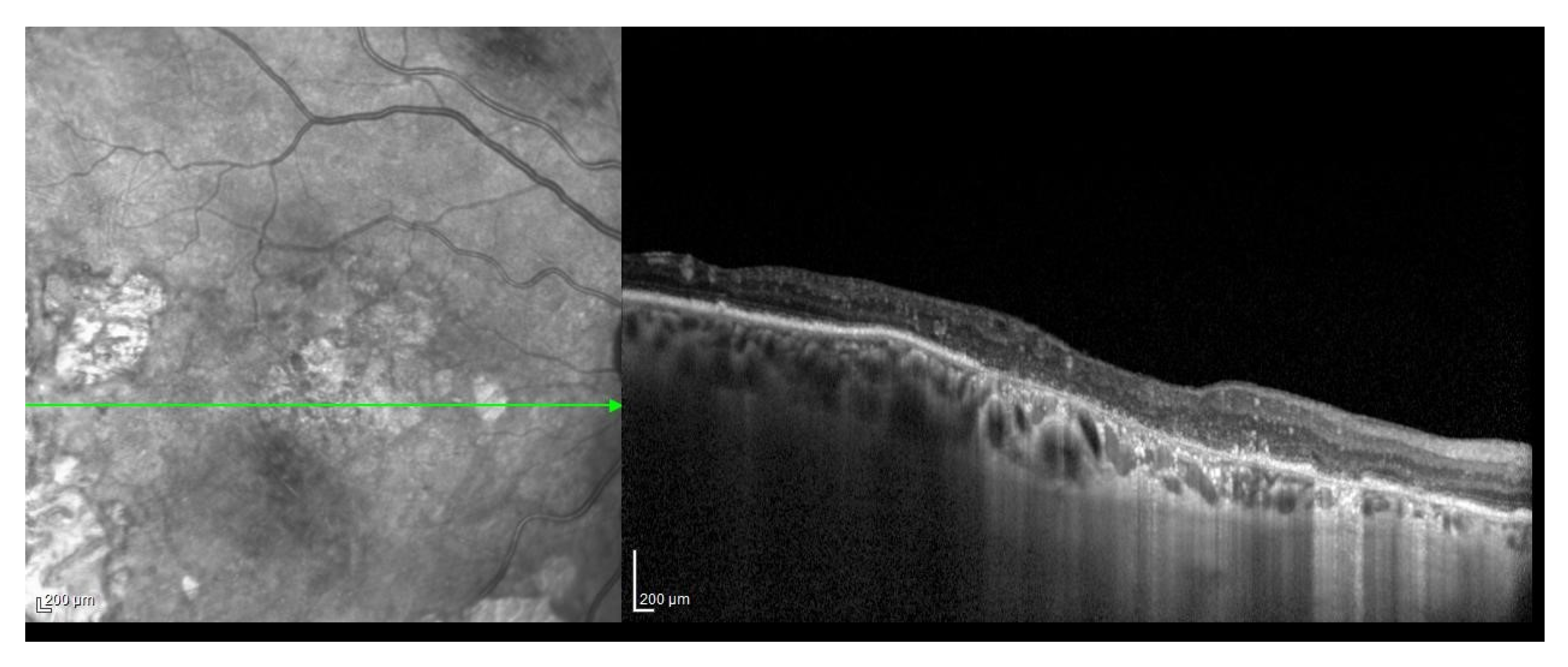
2.2. ARMD Case Definition and Grading
2.3. Periodontal Disease Case Definition and Staging
2.4. Medical Data Collection
2.5. Data Management and Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Dental and Periodontal Data
3.2.1. Periodontal Parameters in Cases vs. Controls
3.2.2. Periodontal Parameters in Relation to ARMD Risk Factors in Cases
4. Discussion
4.1. Periodontal Parameters in Cases vs. Controls
4.2. Periodontal Parameters in Relation to ARMD Risk Factors in Cases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nowak, J.Z. ARMD-the retinal disease with an unprecisedetio pathogenesis: In search of effective therapeutics. Acta Pol. Pharm. 2014, 71, 900–916. [Google Scholar] [PubMed]
- Bahadorani, S.; Singer, M. Recent advances in the management and understanding of macular degeneration. F1000Res 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, J.Z. Age-related macular degeneration (ARMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar] [PubMed]
- Cho, B.J.; Heo, J.W.; Kim, T.W.; Ahn, J.; Chung, H. Prevalence and risk factors of age related macular degeneration in Korea: The Korea National Health and Nutrition Examination Survey 2010–2011. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Karesvuo, P.; Gursoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Huumonen, S.; Vesti, E.; Könönen, E. Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2013, 84, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.; Klaver, C.C.; Hofman, A.; De Jong, P.T. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005, 294, 3101–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, J.M.; Cote, J.; Davis, N.; Rosner, B. Progression of age-related macular degeneration: Association with body mass index, waist circumference, and waist-hip ratio. Arch. Ophthalmol. 2003, 121, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Moeini, H.A.; Masoudpour, H.; Ghanbari, H. A study of the relation between body mass index and the incidence of age related macular degeneration. Br. J. Ophthalmol. 2005, 89, 964–966. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Knudtson, M.D.; Cruickshanks, K.J.; Klein, B.E. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: The Beaver Dam Eye Study. Arch. Ophthalmol. 2008, 126, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Wagley, S.; Marra, K.V.; Salhi, R.A.; Gautam, S.; Campo, R.; Veale, P.; Veale, J.; Arroyo, J.G. Periodontal disease and age-related macular degeneration: Results from the National Health and Nutrition Examination Survey III. Retina 2015, 35, 982–988. [Google Scholar] [CrossRef]
- Shin, Y.U.; Lim, H.W.; Hong, E.H.; Kang, M.H.; Seong, M.; Nam, E.; Cho, H. The association between periodontal disease and age-related macular degeneration in the Korea National health and nutrition examination survey: A cross-sectional observational study. Medicine 2017, 96, e6418. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45, 130–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, L.; Di Spirito, F.; Sirignano, M.; La Rocca, M.; Esposito, U.; Sbordone, L. A 5-year longitudinal cohort study on crown to implant ratio effect on marginal bone level in single implants. Clin. Implant Dent. Relat. 2019, 21, 916–922. [Google Scholar] [CrossRef]
- Di Spirito, F.; Sbordone, L.; Pilone, V.; D’Ambrosio, F. Obesity and periodontal disease: A narrative review on current evidences and putative molecular links. Open. Dent. J. 2019, 13, 526–536. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Pilone, V.; Carinci, F.; Lauritano, D.; Sbordone, L. The association between periodontitis and human colorectal cancer: Genetic and pathogenic linkage. Life 2020, 10, 211. [Google Scholar] [CrossRef]
- Figuero, E.; Sánchez-Beltrán, M.; Cuesta-Frechoso, S.; Tejerina, J.M.; Del Castro, J.A.; Gutiérrez, J.M.; Herrera, D.; Sanz, M. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J. Periodontol. 2011, 82, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Gregori, G.; Wang, F.; Rosenfeld, P.J.; Yehoshua, Z.; Gregori, N.Z.; Lujan, B.J.; Puliafito, C.A.; Feuer, W.J. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology 2011, 118, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Löe, H.; Silness, J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Löe, H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Tonetti, M.S. Periodontal diagnosis in treated periodontitis. Why, when and how to use clinical parameters. J. Clin. Periodontol. 1996, 23, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Barone, A.; Chatelain, S.; Derchi, G.; Di Spirito, F.; Martuscelli, R.; Porzio, M.; Sbordone, L. Effectiveness of antibiotics in preventingalveolitisaftereruptedtooth extraction: A retrospectivestudy. Oral. Dis. 2020, 26, 967–973. [Google Scholar] [CrossRef]
- Wilcox, R.; Tian, T. Measuring effect size: A robust heteroscedastic approach for two or more groups. J. Appl. Stat. 2011, 38, 1359–1368. [Google Scholar] [CrossRef]
- Luh, W.M.; Guo, J.H. The sample size needed for the trimmed t-test when one group size is fixed. J. Exp. Educ. 2010, 78, 14–25. [Google Scholar] [CrossRef]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia Pac. J. Ophthalmol. (Phila.) 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Brzozowska, A.; Puchalska-Niedba, L. Oral status as a potential source of infection in ARMD patients-introduction. Klin. Oczna 2012, 114, 29–32. [Google Scholar] [PubMed]
- De Bernardo, M.; Rosa, N. Letters to the editor: Re: Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2014, 85, 11–12. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-Tumanyan, S.; Spiegelman, D.; Curhan, G.C.; Curhan, G.C.; Forman, J.P.; Joshipura, K.J. Periodontal disease and incidence of hypertension in the health professionals follow-up study. Am. J. Hypertens. 2012, 25, 770–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, K.K.; Seddon, J.M. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthal. Epidemiol. 1999, 6, 125–143. [Google Scholar] [CrossRef] [PubMed]
- D’aiuto, F.; Sabbah, W.; Netuveli, G.; Donos, N.; Hingorani, A.D.; Deanfield, J.; Tsakos, G. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J. Clin. Endocrinol. Metab. 2008, 93, 3989–3994. [Google Scholar] [CrossRef] [Green Version]
- Völzke, H.; Sabbah, W.; Netuveli, G.; Donos, N.; Hingorani, A.D.; Deanfield, J.; Tsakos, G. Gender differences in the relation between number of teeth and systolic blood pressure. J. Hypertens. 2006, 24, 1257–1263. [Google Scholar] [CrossRef]
- Taguchi, A.; Sanada, M.; Suei, Y.; Ohtsuka, M.; Lee, K.; Tanimoto, K.; Tsuda, M.; Ohama, K.; Yoshizumi, M.; Higashi, Y. Tooth loss is associated with an increased risk of hypertension in postmenopausal women. Hypertension 2004, 43, 1297–1300. [Google Scholar] [CrossRef] [Green Version]
- Joshipura, K.J.; Hung, H.C.; Rimm, E.B.; Willett, W.C.; Ascherio, A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke 2003, 34, 47–52. [Google Scholar] [CrossRef] [Green Version]
- De Stefano, F.; Anda, R.F.; Kahn, H.S.; Williamson, D.F.; Russell, C.M. Dental disease and risk of coronary heart disease and mortality. BMJ 1993, 306, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Lowe, G.; Woodward, M.; Rumley, A.; Morrison, C.; Tunstall-Pedoe, H.; Stephen, K. Total tooth loss and prevalent cardiovascular disease in men and women: Possible roles of citrusfruit consumption, vitamin C, and inflammatory and thrombotic variables. J. Clin. Epidemiol. 2003, 56, 694–700. [Google Scholar] [CrossRef]
- Zhu, Y.; Hollis, J.H. Tooth loss and its association with dietary intake and diet quality in American adults. J. Dent. 2014, 42, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, C.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Di Spirito, F. Computed tomography-aided descriptive analysis of maxillary and mandibular atrophies. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Pockpa, Z.A.D.; Struillou, X.; Kone, D.; Mobio, G.S.; Soueidan, A.; Badran, Z. Periodontal Diseases and Age-Related Macular Degeneration: Is There a Link? A Review. Perm. J. 2019, 23. [Google Scholar] [CrossRef]
- Xuewen, L.; Weiqi, L.; Zhiyu, F.; Xiaofei, X.; Chunling, P. Periodontal Disease and Age-Related Macular Degeneration: A Meta-Analysis of 112,240 Participants. Biomed. Res. Int. 2020, 2020, 4753645. [Google Scholar] [CrossRef]

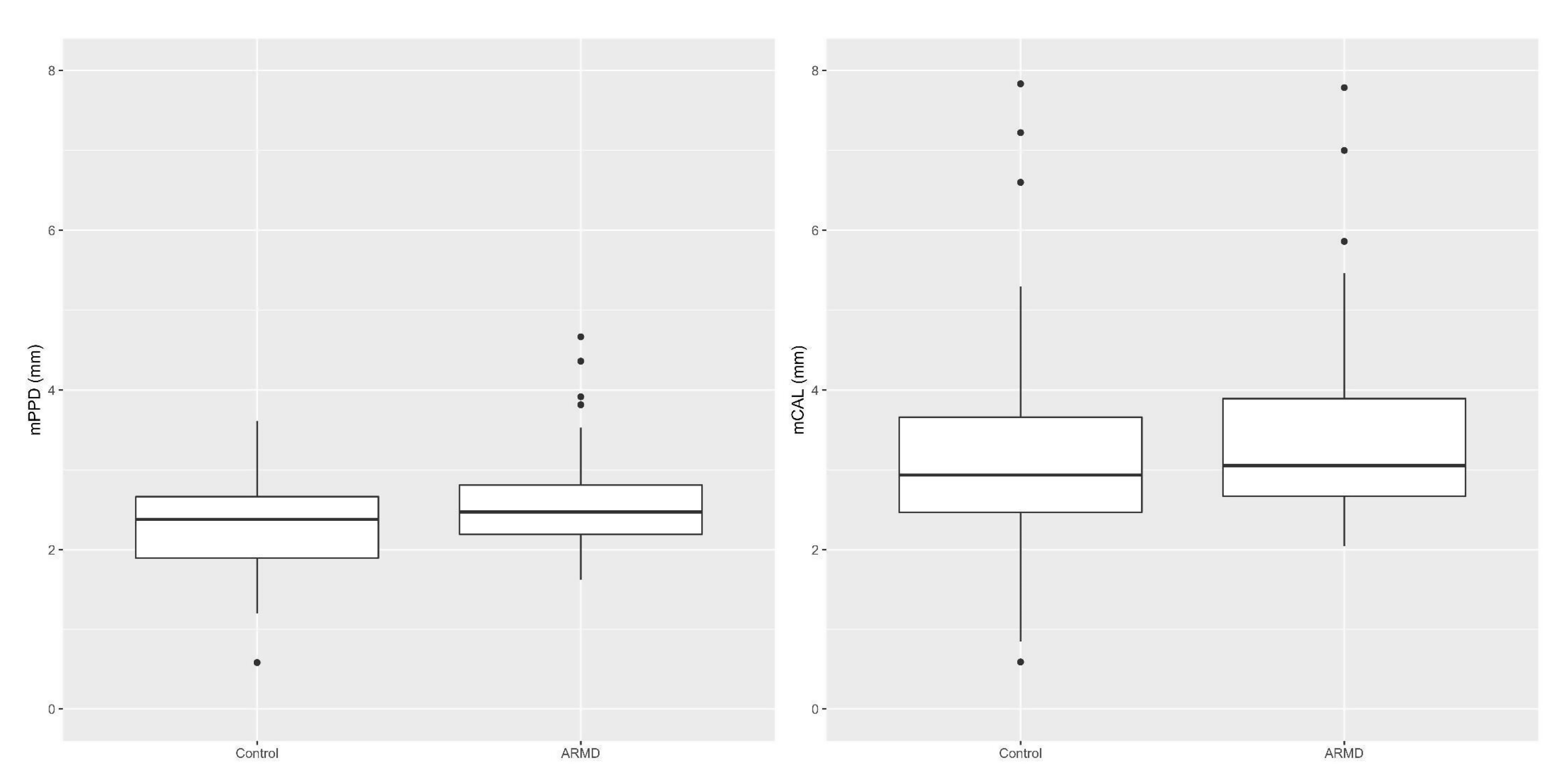

| Mean Teeth Number (±SD*) | Mean CAL (±SD*) | Mean PPD (±SD*) | FMBS% (±SD*) | FMPS% (±SD*) | |
|---|---|---|---|---|---|
| Cases | 17.48 (±7.73) | 3.48 (±1.28) | 2.63 (±0.70) | 94.1% (±0.19) | 93.9% (±0.18) |
| Controls | 17.33 (±6.68) | 3.19 (±1.56) | 2.34 (±0.64) | 74.4% (±0.37) | 81.3% (±0.31) |
| CAL Values at the Site of Greatest Loss | Cases n. (%) | Controls n. (%) |
|---|---|---|
| 1 to 2 mm | 0(0%) | 0(0%) |
| 3 to 4 mm | 2(5%) | 2(5%) |
| ≥5 mm | 38 (95%) | 38(95%) |
| Alveolar Bone Loss (Bone Pocket) As per Karesvuoet al. 2013 | Cases n. (%) | Controls n. (%) |
|---|---|---|
| Class 0 No bone pocket | 5 (12.5%) | 6 (15%) |
| Class 1 Bone pocket exceeding the middle third of the root | 26 (65%) | 24 (60%) |
| Class 2 Bone pocket exceeding the apical third of the root | 9 (22.5%) | 10 (25%) |
| Radiographic Bone Loss (RBL) As per Tonetti et al. (2018) | Cases n. (%) | Controls n. (%) |
| Coronal third (<15%) | 2 (5%) | 3 (7.5%) |
| Coronal third (15% to 33%) | 3 (7.5%) | 3 (7.5%) |
| Extending to the middle or apical third of the root (>33%) | 35 (87.5%) | 34 (85%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2021, 9, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9010001
Di Spirito F, La Rocca M, De Bernardo M, Rosa N, Sbordone C, Sbordone L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dentistry Journal. 2021; 9(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9010001
Chicago/Turabian StyleDi Spirito, Federica, Michele La Rocca, Maddalena De Bernardo, Nicola Rosa, Carolina Sbordone, and Ludovico Sbordone. 2021. "Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study" Dentistry Journal 9, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9010001





