In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
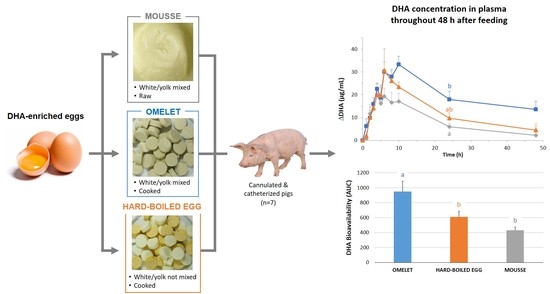
2.2. DHA-Enriched Test Meals
2.3. Animals and Animal Housing
2.4. Experimental and Sampling Procedures
2.5. Chemical Analyses
2.5.1. Lipid Extraction from Food Matrices
2.5.2. DHA Quantification in Food Matrices, Effluents, and Plasma
2.5.3. Protein Concentration and Proteolysis in Effluents
2.6. Statistical Analyses
3. Results
3.1. DHA Recovery in the Matrices
3.2. De Visu Morphological Characterization of the Duodenal Effluents
3.3. Physicochemical Evolution of the Duodenal Effluents throughout the Postprandial Period
3.3.1. pH Evolution
3.3.2. Evolution of Protein Concentration
3.3.3. Evolution of Proteolysis
3.3.4. Evolution of DHA Concentration
3.4. Evolution of the Plasma Concentration of DHA throughout the Postprandial Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stillwell, W.; Wassall, S.R. Docosahexaenoic Acid: Membrane Properties of a Unique Fatty Acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Innis, S.M.; Friesen, R.W. Essential N-3 Fatty Acids in Pregnant Women and Early Visual Acuity Maturation in Term Infants. Am. J. Clin. Nutr. 2008, 87, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, M.A.; Broadhurst, C.L.; Guest, M.; Nagar, A.; Wang, Y.; Ghebremeskel, K.; Schmidt, W.F. A Quantum Theory for the Irreplaceable Role of Docosahexaenoic Acid in Neural Cell Signalling throughout Evolution. Prostag. Leukotr. Essent. Fat. Acids 2013, 88, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of Omega-3 Fatty Acids in Obesity, Metabolic Syndrome, and Cardiovascular Diseases: A Review of the Evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, J.; Rossmeisl, M.; Flachs, P.; Kuda, O.; Brauner, P.; Jilkova, Z.; Stankova, B.; Tvrzicka, E.; Bryhn, M. N-3 PUFA: Bioavailability and Modulation of Adipose Tissue Function: Symposium on ‘Frontiers in Adipose Tissue Biology. Proc. Nutr. Soc. 2009, 68, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, A.M.; Ding, E.L.; Willett, W.C.; Rimm, E.B. A Meta-Analysis Shows That Docosahexaenoic Acid from Algal Oil Reduces Serum Triglycerides and Increases HDL-Cholesterol and LDL-Cholesterol in Persons without Coronary Heart Disease. J. Nutr. 2012, 142, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Dawczynski, C.; Martin, L.; Wagner, A.; Jahreis, G. N-3 LC-PUFA-Enriched Dairy Products Are Able to Reduce Cardiovascular Risk Factors: A Double-Blind, Cross-over Study. Clin. Nutr. 2010, 29, 592–599. [Google Scholar] [CrossRef]
- Warner, J.G.; Ullrich, I.H.; Albrink, M.J.; Yeater, R.A. Combined Effects of Aerobic Exercise and Omega-3 Fatty Acids in Hyperlipidemic Persons. Med. Sci. Sports Exerc. 1989, 21, 498–505. [Google Scholar] [CrossRef]
- Simão, A.N.C.; Lozovoy, M.A.B.; Bahls, L.D.; Morimoto, H.K.; Simão, T.N.C.; Matsuo, T.; Dichi, I. Blood Pressure Decrease with Ingestion of a Soya Product (Kinako) or Fish Oil in Women with the Metabolic Syndrome: Role of Adiponectin and Nitric Oxide. Br. J. Nutr. 2012, 108, 1435–1442. [Google Scholar] [CrossRef]
- Ramel, A.; Martinez, J.A.; Kiely, M.; Bandarra, N.M.; Thorsdottir, I. Moderate Consumption of Fatty Fish Reduces Diastolic Blood Pressure in Overweight and Obese European Young Adults during Energy Restriction. Nutrition 2010, 26, 168–174. [Google Scholar] [CrossRef]
- Axelrod, L.; Camuso, J.; Williams, E.; Kleinman, K.; Briones, E.; Schoenfeld, D. Effects of a Small Quantity of Omega-3 Fatty Acids on Cardiovascular Risk Factors in NIDDM. A Randomized, Prospective, Double-Blind, Controlled Study. Diabetes Care 1994, 17, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.A.; Raskin, P.; Rosenstock, J. A Comparison of Fish Oil or Corn Oil Supplements in Hyperlipidemic Subjects with NIDDM. Diabetes Care 1995, 18, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciubotaru, I.; Lee, Y.-S.; Wander, R.C. Dietary Fish Oil Decreases C-Reactive Protein, Interleukin-6, and Triacylglycerol to HDL-Cholesterol Ratio in Postmenopausal Women on HRT. J. Nutr. Biochem. 2003, 14, 513–521. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Rondó, P.H.C. Omega-3 Fatty Acids and Hypertriglyceridemia in HIV-Infected Subjects on Antiretroviral Therapy: Systematic Review and Meta-Analysis. HIV Clin. Trials 2011, 12, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The Differential Effects of EPA and DHA on Cardiovascular Risk Factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedor, D.; Kelley, D.S. Prevention of Insulin Resistance by N-3 Polyunsaturated Fatty Acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Véricel, E.; Polette, A.; Bacot, S.; Calzada, C.; Lagarde, M. Pro- and Antioxidant Activities of Docosahexaenoic Acid on Human Blood Platelets. J. Thromb. Haemost. 2003, 1, 566–572. [Google Scholar] [CrossRef]
- Yagi, S.; Aihara, K.; Fukuda, D.; Takashima, A.; Hara, T.; Hotchi, J.; Ise, T.; Yamaguchi, K.; Tobiume, T.; Iwase, T.; et al. Effects of Docosahexaenoic Acid on the Endothelial Function in Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2015, 22, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic Acid Induces an Anti-Inflammatory Profile in Lipopolysaccharide-Stimulated Human THP-1 Macrophages More Effectively than Eicosapentaenoic Acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Nakamura, N.; Hamazaki, T.; Kobayashi, M.; Yazawa, K. The Effect of Oral Administration of Eicosapentaenoic and Docosahexaenoic Acids on Acute Inflammation and Fatty Acid Composition in Rats. J. Nutr. Sci. Vitaminol. 1994, 40, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Horrocks, L.A.; Yeo, Y.K. Health Benefits of Docosahexaenoic Acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapkin, R.S.; McMurray, D.N.; Davidson, L.A.; Patil, B.S.; Fan, Y.-Y.; Lupton, J.R. Bioactive Dietary Long-Chain Fatty Acids: Emerging Mechanisms of Action. Br. J. Nutr. 2008, 100, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Tan, L.; Wang, C.; Ma, J.; Li, N.; Li, Y.; Xu, G.; Li, J. Docosahexaenoic Acid Changes Lipid Composition and Interleukin-2 Receptor Signaling in Membrane Rafts. J. Lipid Res. 2005, 46, 1904–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.P.; Connolly, J.M.; Rayburn, J.; Coleman, M. Influence of Diets Containing Eicosapentaenoic or Docosahexaenoic Acid on Growth and Metastasis of Breast Cancer Cells in Nude Mice. J. Natl. Cancer Inst. 1995, 87, 587–592. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Dahlén, S.E.; Björk, J.; Hedqvist, P.; Arfors, K.E.; Hammarström, S.; Lindgren, J.A.; Samuelsson, B. Leukotrienes Promote Plasma Leakage and Leukocyte Adhesion in Postcapillary Venules: In Vivo Effects with Relevance to the Acute Inflammatory Response. Proc. Natl. Acad. Sci. USA 1981, 78, 3887–3891. [Google Scholar] [CrossRef] [Green Version]
- Hodge, L.; Salome, C.M.; Hughes, J.M.; Liu-Brennan, D.; Rimmer, J.; Allman, M.; Pang, D.; Armour, C.; Woolcock, A.J. Effect of Dietary Intake of Omega-3 and Omega-6 Fatty Acids on Severity of Asthma in Children. Eur. Respir. J. 1998, 11, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Dangour, A.D.; Whitehouse, P.J.; Rafferty, K.; Mitchell, S.A.; Smith, L.; Hawkesworth, S.; Vellas, B. B-Vitamins and Fatty Acids in the Prevention and Treatment of Alzheimer’s Disease and Dementia: A Systematic Review. J. Alzheimers Dis. 2010, 22, 205–224. [Google Scholar] [CrossRef] [Green Version]
- Sydenham, E.; Dangour, A.D.; Lim, W.-S. Omega 3 Fatty Acid for the Prevention of Cognitive Decline and Dementia. Cochrane Database Syst. Rev. 2012, CD005379. [Google Scholar] [CrossRef] [Green Version]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Launer, L.J.; Grodstein, F.; Bernstein, P.S. Age-Related Eye Disease Study 2 (AREDS2) Research Group. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. J. Am. Med. Assoc. 2015, 314, 791–801. [Google Scholar] [CrossRef]
- Tully, A.M.; Roche, H.M.; Doyle, R.; Fallon, C.; Bruce, I.; Lawlor, B.; Coakley, D.; Gibney, M.J. Low Serum Cholesteryl Ester-Docosahexaenoic Acid Levels in Alzheimer’s Disease: A Case-Control Study. Br. J. Nutr. 2003, 89, 483–489. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish Consumption, n-3 Fatty Acids, and Subsequent 5-y Cognitive Decline in Elderly Men: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.A.; Pynaert, I.; Matthys, C.; De Backer, G.; Van Camp, J.; De Henauw, S. Dietary Intakes and Food Sources of Fatty Acids for Belgian Women, Focused on n-6 and n-3 Polyunsaturated Fatty Acids. Lipids 2006, 41, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, N.; Eggersdorfer, M. Is the World Supply of Omega-3 Fatty Acids Adequate for Optimal Human Nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States during the 20th Century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.J. Are We Consuming Enough Long Chain Omega-3 Polyunsaturated Fatty Acids for Optimal Health? Prostag. Leukotr. Essent. Fat. Acids 2011, 85, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: A Guideline from the American Heart Association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [Green Version]
- Aranceta, J.; Pérez-Rodrigo, C. Recommended Dietary Reference Intakes, Nutritional Goals and Dietary Guidelines for Fat and Fatty Acids: A Systematic Review. Br. J. Nutr. 2012, 107 (Suppl. S2), S8–S22. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of Conversion of Alpha-Linolenic Acid to Long Chain n-3 Fatty Acids in Man. Curr. Opin. Clin. Nutr. 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Goyens, P.L.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of Alpha-Linolenic Acid in Humans Is Influenced by the Absolute Amounts of Alpha-Linolenic Acid and Linoleic Acid in the Diet and Not by Their Ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Samman, S.; Kung, F.P.; Carter, L.M.; Foster, M.J.; Ahmad, Z.I.; Phuyal, J.L.; Petocz, P. Fatty Acid Composition of Certified Organic, Conventional and Omega-3 Eggs. Food Chem. 2009, 116, 911–914. [Google Scholar] [CrossRef]
- Cunningham, E. Are Krill Oil Supplements a Better Source of N-3 Fatty Acids than Fish Oil Supplements? J. Acad. Nutr. Diet. 2012, 112, 344. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Holven, K.B. Comparison of Bioavailability of Krill Oil versus Fish Oil and Health Effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destaillats, F.; Oliveira, M.; Bastic Schmid, V.; Masserey-Elmelegy, I.; Giuffrida, F.; Thakkar, S.K.; Dupuis, L.; Gosoniu, M.L.; Cruz-Hernandez, C. Comparison of the Incorporation of DHA in Circulatory and Neural Tissue When Provided as Triacylglycerol (TAG), Monoacylglycerol (MAG) or Phospholipids (PL) Provides New Insight into Fatty Acid Bioavailability. Nutrients 2018, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Apriani, C.; Weerakkody, R.; Sanguansri, L.; Augustin, M.A. Food Matrix Effects on in Vitro Digestion of Microencapsulated Tuna Oil Powder. J. Agric. Food Chem. 2011, 59, 8442–8449. [Google Scholar] [CrossRef]
- Schram, L.B.; Nielsen, C.J.; Porsgaard, T.; Nielsen, N.S.; Holm, R.; Mu, H. Food Matrices Affect the Bioavailability of (N−3) Polyunsaturated Fatty Acids in a Single Meal Study in Humans. Food Res. Int. 2007, 40, 1062–1068. [Google Scholar] [CrossRef]
- Sauveur, B. Reproduction des Volailles et Production D’oeufs; Quae: Paris, France, 1988. [Google Scholar]
- Hernell, O.; Staggers, J.E.; Carey, M.C. Physical-Chemical Behavior of Dietary and Biliary Lipids during Intestinal Digestion and Absorption. 2. Phase Analysis and Aggregation States of Luminal Lipids during Duodenal Fat Digestion in Healthy Adult Human Beings. Biochemistry 1990, 29, 2041–2056. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Lopez, C.; Briard-Bion, V.; Menard, O.; Rousseau, F.; Pradel, P.; Besle, J.-M. Phospholipid, Sphingolipid, and Fatty Acid Compositions of the Milk Fat Globule Membrane Are Modified by Diet. J. Agric. Food Chem. 2008, 56, 5226–5236. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using O-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins1. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. NparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. Nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.S.; Gabert, V.M.; Jørgensen, H.; Engberg, R.M. Collection of Pancreatic Juice from Growing Pigs. Int. J. Pancreatol. 1997, 21, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, T.; Low, A.G.; Zebrowska, H. Studies on Gastric Digestion of Protein and Carbohydrate, Gastric Secretion and Exocrine Pancreatic Secretion in the Growing Pig. Br. J. Nutr. 1983, 49, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, T.; Low, A.G. The Influence of Diets Based on Whole Wheat, Wheat Flour and Wheat Bran on Exocrine Pancreatic Secretion in Pigs. J. Nutr. 1987, 117, 1212–1216. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and Bioaccessibility of Food Bioactive Compounds; Overview and Assessment by in Vitro Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Adeola, O.; Xue, P.C.; Cowieson, A.J.; Ajuwon, K.M. Basal Endogenous Losses of Amino Acids in Protein Nutrition Research for Swine and Poultry. Anim. Feed Sci. Tech. 2016, 221, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lapenna, D.; Ciofani, G. Chromium and Human Low-Density Lipoprotein Oxidation. J. Trace Elem. Med. Bio. 2020, 59, 126411. [Google Scholar] [CrossRef] [PubMed]
- Nau, F.; Nyemb-Diop, K.; Lechevalier, V.; Floury, J.; Serrière, C.; Stroebinger, N.; Boucher, T.; Guérin-Dubiard, C.; Ferrua, M.J.; Dupont, D.; et al. Spatial-Temporal Changes in PH, Structure and Rheology of the Gastric Chyme in Pigs as Influenced by Egg White Gel Properties. Food Chem. 2019, 280, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Ménard, O.; Le Gouar, Y.; Buffière, C.; Famelart, M.-H.; Laroche, B.; Le Feunteun, S.; Dupont, D.; Rémond, D. The Heat Treatment and the Gelation Are Strong Determinants of the Kinetics of Milk Proteins Digestion and of the Peripheral Availability of Amino Acids. Food Chem. 2013, 136, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M.; Grybäck, P.; Jacobsson, H. The Physiology of Gastric Emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R. Disintegration of Solid Foods in Human Stomach. J. Food Sci. 2008, 73, R67–R80. [Google Scholar] [CrossRef]
- Thévenot, J.; Cauty, C.; Legland, D.; Dupont, D.; Floury, J. Pepsin Diffusion in Dairy Gels Depends on Casein Concentration and Microstructure. Food Chem. 2017, 223, 54–61. [Google Scholar] [CrossRef]
- Luo, Q.; Borst, J.W.; Westphal, A.H.; Boom, R.M.; Janssen, A.E.M. Pepsin Diffusivity in Whey Protein Gels and Its Effect on Gastric Digestion. Food Hydrocoll. 2017, 66, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Somaratne, G.; Nau, F.; Ferrua, M.J.; Singh, J.; Ye, A.; Dupont, D.; Singh, R.P.; Floury, J. Characterization of Egg White Gel Microstructure and Its Relationship with Pepsin Diffusivity. Food Hydrocoll. 2020, 98, 105258. [Google Scholar] [CrossRef]
- Floury, J.; Bianchi, T.; Thévenot, J.; Dupont, D.; Jamme, F.; Lutton, E.; Panouillé, M.; Boué, F.; Le Feunteun, S. Exploring the Breakdown of Dairy Protein Gels during in Vitro Gastric Digestion Using Time-Lapse Synchrotron Deep-UV Fluorescence Microscopy. Food Chem. 2018, 239, 898–910. [Google Scholar] [CrossRef]
- Carlier, H.; Bernard, A.; Caselli, C. Digestion and Absorption of Polyunsaturated Fatty Acids. Reprod. Nutr. Dev. 1991, 31, 475–500. [Google Scholar] [CrossRef]
- Trotter, P.J.; Ho, S.Y.; Storch, J. Fatty Acid Uptake by Caco-2 Human Intestinal Cells. J. Lipid Res. 1996, 37, 336–346. [Google Scholar] [PubMed]
- Abumrad, N.A.; Park, J.H.; Park, C.R. Permeation of Long-Chain Fatty Acid into Adipocytes. Kinetics, Specificity, and Evidence for Involvement of a Membrane Protein. J. Biol. Chem. 1984, 259, 8945–8953. [Google Scholar] [PubMed]
- Stremmel, W.; Strohmeyer, G.; Berk, P.D. Hepatocellular Uptake of Oleate Is Energy Dependent, Sodium Linked, and Inhibited by an Antibody to a Hepatocyte Plasma Membrane Fatty Acid Binding Protein. Proc. Natl. Acad. Sci. USA 1986, 83, 3584–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrentino, D.; Robinson, R.B.; Kiang, C.L.; Berk, P.D. At Physiologic Albumin/Oleate Concentrations Oleate Uptake by Isolated Hepatocytes, Cardiac Myocytes, and Adipocytes Is a Saturable Function of the Unbound Oleate Concentration. Uptake Kinetics Are Consistent with the Conventional Theory. J. Clin. Investig. 1989, 84, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Lewis, G.F. New Insights into the Regulation of Chylomicron Production. Annu. Rev. Nutr. 2015, 35, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Boller, M.; Medina, I.; Storch, J. Relative Levels of Dietary EPA and DHA Impact Gastric Oxidation and Essential Fatty Acid Uptake. J. Nutr. Biochem. 2018, 55, 68–75. [Google Scholar] [CrossRef]
- Al-Mashhadi, R.H.; Sørensen, C.B.; Kragh, P.M.; Christoffersen, C.; Mortensen, M.B.; Tolbod, L.P.; Thim, T.; Du, Y.; Li, J.; Liu, Y.; et al. Familial Hypercholesterolemia and Atherosclerosis in Cloned Minipigs Created by DNA Transposition of a Human PCSK9 Gain-of-Function Mutant. Sci. Transl. Med. 2013, 5, 166ra1. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.; Fehlings, C.; Herbach, N.; Hofmann, A.; von Waldthausen, D.C.; Kessler, B.; Ulrichs, K.; Chodnevskaja, I.; Moskalenko, V.; Amselgruber, W.; et al. Glucose Intolerance and Reduced Proliferation of Pancreatic Beta-Cells in Transgenic Pigs with Impaired Glucose-Dependent Insulinotropic Polypeptide Function. Diabetes 2010, 59, 1228–1238. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.H.; Yong, H.Y.; Murphy, C.N.; Wax, D.; Samuel, M.; Rieke, A.; Lai, L.; Liu, Z.; Durtschi, D.C.; Welbern, V.R.; et al. Production of Endothelial Nitric Oxide Synthase (ENOS) over-Expressing Piglets. Transgenic Res. 2006, 15, 739–750. [Google Scholar] [CrossRef]






| Common Name | Chemical Name | Formula | mg/g of Product | Relative % Total Fatty Acids (w/w) |
|---|---|---|---|---|
| Myristic acid | Tetradecanoic acid | C 14:0 | 2.29 | 0.41 |
| Valeric acid | Pentanoic acid | C 15:0 | 0.83 | 0.15 |
| Palmitic acid | Hexadecanoic acid | C 16:0 | 148.72 | 26.85 |
| Palmitoleic acid | 9-cis-Hexadecenoic acid | C 16:1 | 22.33 | 4.03 |
| Margaric acid | Heptadecanoic acid | C 17:0 | 0.62 | 0.11 |
| Stearic acid | Octadecanoic acid | C 18:0 | 38.14 | 6.89 |
| Oleic acid | cis-9-Octadecenoic acid | C 18:1 | 243.09 | 43.89 |
| Linoleic acid | all-cis-9,12-octadecadienoic acid | C 18:2 (n-6) | 52.23 | 9.43 |
| ɣ-linolenic acid, GLA | all-cis-6,9,12-octadecatrienoic acid | C 18:3 (n-6) | 0.32 | 0.06 |
| α-linolenic acid, ALA | all-cis-9,12,15-octadecatrienoic acid | C 18:3 (n-3) | 2.45 | 0.44 |
| Eicosenoic acid | cis-11-eicosenoic acid | C 20:1 | 1.38 | 0.25 |
| Dihomo-ɣ-linolenic acid, DGLA | all-cis-8,11,14-eicosatrienoic acid | C 20:3 (n-6) | 0.30 | 0.05 |
| Arachidonic acid, AA | all-cis-5,8,11,14-eicosatetraenoic acid | C 20:4 (n-6) | 3.46 | 0.62 |
| Timnodonic acid, EPA | all-cis-5,8,11,14,17-eicosapentaenoic acid | C 20:5 (n-3) | 1.85 | 0.33 |
| Clupanodonic acid, DPA | all-cis-7,10,13,16,19-docosapentaenoic acid | C 22:5 (n-3) | 1.57 | 0.28 |
| Osbond acid | all-cis-4,7,10,13,16-docosapentaenoic acid | C 22:5 (n-6) | 0.42 | 0.08 |
| Cervonic acid, DHA | all-cis-4,7,10,13,16,19-docosahexaenoic acid | C 22:6 (n-3) | 26.25 | 4.74 |
| Total mg | 553.92 |
| Omelet | Hard-Boiled Egg | Mousse |
|---|---|---|
| 99.4 ± 2.5 | 95.13 ± 0.0 | 91.7 ± 3.6 |
| Omelet | Hard-Boiled Egg | Mousse | |
|---|---|---|---|
| AUC (calculated from Figure 6) | 950.1 ± 140 a | 609.5 ± 78.6 b | 432.5 ± 43 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Vadillo, C.; Nau, F.; Guérin-Dubiard, C.; Bourlieu, C.; Capozzi, F.; Bordoni, A.; Dupont, D. In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability. Foods 2021, 10, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010006
Pineda-Vadillo C, Nau F, Guérin-Dubiard C, Bourlieu C, Capozzi F, Bordoni A, Dupont D. In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability. Foods. 2021; 10(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010006
Chicago/Turabian StylePineda-Vadillo, Carlos, Françoise Nau, Catherine Guérin-Dubiard, Claire Bourlieu, Francesco Capozzi, Alessandra Bordoni, and Didier Dupont. 2021. "In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability" Foods 10, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010006