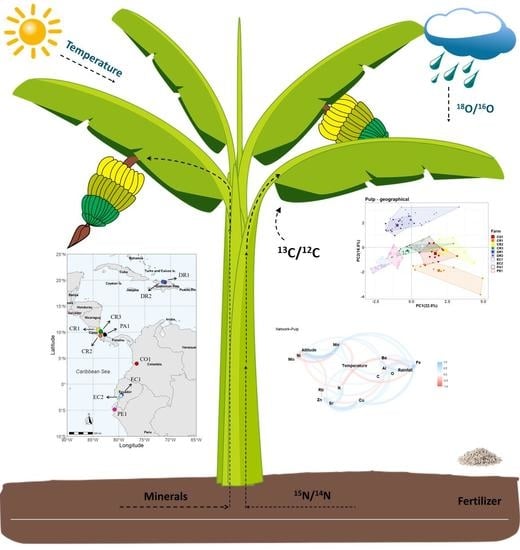
Preliminary Study on Tracing the Origin and Exploring the Relations between Growing Conditions and Isotopic and Elemental Fingerprints of Organic and Conventional Cavendish Bananas (Musa spp.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Growing Conditions
2.2. Determining the Stable Isotope Ratios
2.3. Determining the Elemental Compositions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Summary of the Growing Conditions of Banana Farms
3.2. Differences of Stable Isotopes between Bananas from Different Growing Conditions
3.3. Differences of Elemental Compositions between Bananas from Different Growing Conditions
3.4. Principal Component Analysis of Bananas between the Different Growing Condition
3.5. Correlation Analysis of Banana Compositions and Their Growing Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyeyinka, B.O.; Afolayan, A.J. Comparative Evaluation of the Nutritive, Mineral, and Antinutritive Composition of Musa sinensis L. (Banana) and Musa paradisiaca L. (Plantain) Fruit Compartments. Plants 2019, 8, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Dale, J.; James, A.; Paul, J.-Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Garcia-Bastidas, F.; Kema, G.; Waterhouse, P.; Mengersen, K.; et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.; Maraschin, M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef]
- FAO. Banana Market Review 2019; FAO: Roma, Italy, 2020. [Google Scholar]
- Aprile, M.C.; Caputo, V.; Nayga, R.M.N., Jr. Consumers’ valuation of food quality labels: The case of the European geographic indication and organic farming labels. Int. J. Consum. Stud. 2012, 36, 158–165. [Google Scholar] [CrossRef]
- Bellamy, A.S. Banana Production Systems: Identification of Alternative Systems for More Sustainable Production. Ambio 2013, 42, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santeramo, F.G.; Lamonaca, E. Evaluation of geographical label in consumers’ decision-making process: A systematic review and meta-analysis. Food Res. Int. 2020, 131, 108995. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Guo, B.; Wei, Y. Origin assignment by multi-element stable isotopes of lamb tissues. Food Chem. 2016, 213, 675–681. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, S.; Zhang, F.; Huang, M.; Xie, J. Characterization and authentication of olive, camellia and other vegetable oils by combination of chromatographic and chemometric techniques: Role of fatty acids, tocopherols, sterols and squalene. Eur. Food Res. Technol. 2021, 247, 411–426. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef]
- Robson, K.; Dean, M.; Haughey, S.; Elliott, C. A comprehensive review of food fraud terminologies and food fraud mitigation guides. Food Control 2021, 120, 107516. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Luning, P.A.; Silvis, I.C.J.; Yang, Y.; Huisman, W. Differences in fraud vulnerability in various food supply chains and their tiers. Food Control 2018, 84, 375–381. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Huisman, W.; Luning, P.A. Food fraud vulnerability and its key factors. Trends Food Sci. Technol. 2017, 67, 70–75. [Google Scholar] [CrossRef]
- Bronzi, B.; Brilli, C.; Beone, G.M.; Fontanella, M.C.; Ballabio, D.; Todeschini, R.; Consonni, V.; Grisoni, F.; Parri, F.; Buscema, M. Geographical identification of Chianti red wine based on ICP-MS element composition. Food Chem. 2020, 315, 126248. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, T.; Tang, X.; Zhao, S.; Qie, M.; Chen, A.; Yang, S. Authentication of organic pork and identification of geographical origins of pork in four regions of China by combined analysis of stable isotopes and multi-elements. Meat Sci. 2020, 165, 108129. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef]
- Baskali-Bouregaa, N.; Milliand, M.-L.; Mauffrey, S.; Chabert, E.; Forrestier, M.; Gilon, N. Tea geographical origin explained by LIBS elemental profile combined to isotopic information. Talanta 2020, 211, 120674. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Zhang, W.; Zhao, Y.; Yang, S.; Chen, A. Tracing the geographical origin of rice by stable isotopic analyses combined with chemometrics. Food Chem. 2020, 313, 126093. [Google Scholar] [CrossRef]
- De Rijke, E.; Schoorl, J.C.; Cerli, C.; Vonhof, H.B.; Verdegaal, S.J.A.; Vivó-Truyols, G.; Lopatka, M.; Dekter, R.; Bakker, D.; Sjerps, M.J.; et al. The use of δ2H and δ18O isotopic analyses combined with chemometrics as a traceability tool for the geographical origin of bell peppers. Food Chem. 2016, 204, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Geana, I.; Iordache, A.; Ionete, R.; Marinescu, A.; Ranca, A.; Culea, M. Geographical origin identification of Romanian wines by ICP-MS elemental analysis. Food Chem. 2013, 138, 1125–1134. [Google Scholar] [CrossRef]
- Bigot, C.; Bugaud, C.; Camilo, J.; Kapitan, A.; Montet, D.; Meile, J.-C. Impact of farming type, variety and geographical origin on bananas bacterial community. Food Control 2020, 109, 106925. [Google Scholar] [CrossRef]
- Choi, S.-H.; Bong, Y.-S.; Park, J.H.; Lee, K.-S. Geographical origin identification of garlic cultivated in Korea using isotopic and multi-elemental analyses. Food Control 2020, 111, 107064. [Google Scholar] [CrossRef]
- Rees, G.; Kelly, S.D.; Cairns, P.; Ueckermann, H.; Hoelzl, S.; Rossmann, A.; Scotter, M.J. Verifying the geographical origin of poultry: The application of stable isotope and trace element (SITE) analysis. Food Control 2016, 67, 144–154. [Google Scholar] [CrossRef]
- Wang, Z.; Erasmus, S.W.; Dekker, P.; Guo, B.; Stoorvogel, J.J.; Van Ruth, S.M. Linking growing conditions to stable isotope ratios and elemental compositions of Costa Rican bananas (Musa spp.). Food Res. Int. 2020, 129, 108882. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, S.W.; Muller, M.; van der Rijst, M.; Hoffman, L.C. Stable isotope ratio analysis: A potential analytical tool for the authentication of South African lamb meat. Food Chem. 2016, 192, 997–1005. [Google Scholar] [CrossRef]
- Herwig, N.; Stephan, K.; Panne, U.; Pritzkow, W.; Vogl, J. Multi-element screening in milk and feed by SF-ICP-MS. Food Chem. 2011, 124, 1223–1230. [Google Scholar] [CrossRef]
- Yan, J.; Oey, S.B.; van Leeuwen, S.P.J.; van Ruth, S.M. Discrimination of processing grades of olive oil and other vegetable oils by monochloropropanediol esters and glycidyl esters. Food Chem. 2018, 248, 93–100. [Google Scholar] [CrossRef]
- Vera, D.N.; Jiménez-Carvelo, A.M.; Cuadros-Rodríguez, L.; Ruisánchez, I.; Callao, M.P. Authentication of the geographical origin of extra-virgin olive oil of the Arbequina cultivar by chromatographic fingerprinting and chemometrics. Talanta 2019, 203, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pustjens, A.M.; Erasmus, S.W.; Yang, Y.; Hettinga, K.; van Ruth, S.M. Dairy farming system markers: The correlation of forage and milk fatty acid profiles from organic, pasture and conventional systems in the Netherlands. Food Chem. 2020, 314, 126153. [Google Scholar] [CrossRef]
- Laursen, K.H.; Schjoerring, J.K.; Kelly, S.D.; Husted, S. Authentication of organically grown plants—advantages and limitations of atomic spectroscopy for multi-element and stable isotope analysis. TrAC Trends Anal. Chem. 2014, 59, 73–82. [Google Scholar] [CrossRef]
- Laursen, K.H.; Mihailova, A.; Kelly, S.D.; Epov, V.N.; Bérail, S.; Schjoerring, J.K.; Donard, O.F.X.; Larsen, E.H.; Pedentchouk, N.; Marca-Bell, A.D.; et al. Is it really organic? – Multi-isotopic analysis as a tool to discriminate between organic and conventional plants. Food Chem. 2013, 141, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustaffa, M.M.; Kumar, V. Banana production and productivity enhancement through spatial, water and nutrient manage-ment. J. Hortic. Sci. 2012, 7, 1–28. [Google Scholar]
- Emaga, T.H.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Hardisson, A.; Rubio, C.; Baez, A.; Martin, M.; Alvarez, R.; Diaz, E. Mineral composition of the banana (Musa acuminata) from the island of Tenerife. Food Chem. 2001, 73, 153–161. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Zhang, Y.-L.; Song, W.; Lv, Y.-N.; Xu, Q.; Zheng, P.; Zhang, Z.-Z.; Wan, X.-C.; Hou, R.-Y.; Cai, H.-M.; et al. Using stable isotope signatures to delineate the geographic point-of-origin of Keemun black tea. J. Sci. Food Agric. 2019, 99, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, Q.; Zhang, X.; Bao, X.; Wang, Y.; Rasheed, M.; Guo, B. Authentication of the geographical origin of Maca (Lepidium meyenii Walp.) at different regional scales using the stable isotope ratio and mineral elemental fingerprints. Food Chem. 2020, 311, 126058. [Google Scholar] [CrossRef]
- Rapisarda, P.; Camin, F.; Fabroni, S.; Perini, M.; Torrisi, B.; Intrigliolo, F. Influence of Different Organic Fertilizers on Quality Parameters and the δ15N, δ13C, δ2H, δ34S, and δ18O Values of Orange Fruit (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2010, 58, 3502–3506. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, Y.; Xie, T.; Zhang, Y.; Shao, S.; Nie, J.; Xia, W.; Rogers, K.M.; Zhang, W. Long-Term Agricultural Effects on the Authentication Accuracy of Organic, Green, and Conventional Rice Using Isotopic and Elemental Chemometric Analyses. J. Agric. Food Chem. 2020, 68, 1213–1225. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Kernaghan, J.R. Banana production methods. 2014. Available online: https://www.naturskyddsforeningen.se/sites/default/files/dokument-media/banana_report_final_version.pdf (accessed on 4 July 2014).
- Sim, I.; Suh, D.H.; Singh, D.; Do, S.-G.; Moon, K.H.; Lee, J.H.; Ku, K.-M.; Lee, C.H. Unraveling Metabolic Variation for Blueberry and Chokeberry Cultivars Harvested from Different Geo-Climatic Regions in Korea. J. Agric. Food Chem. 2017, 65, 9031–9040. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Liu, J.; Zhang, X.Y.; Wei, X.P.; Qi, Y.; Zhang, B.; Liu, H.; Xiao, P.-G. Metabolomics characterization of different geographical origins of Flos Carthami using UPLC-QTOF/MS and their correlation with climate factors. Anal. Methods 2019, 11, 2700–2711. [Google Scholar] [CrossRef]
- Camin, F.; Dordevic, N.; Wehrens, R.; Neteler, M.; Delucchi, L.; Postma, G.; Buydens, L. Climatic and geographical dependence of the H, C and O stable isotope ratios of Italian wine. Anal. Chim. Acta 2015, 853, 384–390. [Google Scholar] [CrossRef]
- Martin, G.J.; Martin, M.L. Climatic significance of isotope ratios. Phytochem. Rev. 2003, 2, 179–190. [Google Scholar] [CrossRef]
- Coltro, L.; Karaski, T.U. Environmental indicators of banana production in Brazil: Cavendish and Prata varieties. J. Clean. Prod. 2019, 207, 363–378. [Google Scholar] [CrossRef]
- Nishida, M.; Sato, Y. Characteristics of the Relationship between Natural 15N Abundances in Organic Rice and Soil. Plant Prod. Sci. 2015, 18, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, P.; Bordonaba, J.G.; Terry, L.A.; Carlen, C. Characterisation of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem. 2010, 122, 16–24. [Google Scholar] [CrossRef]
- Bennie, J.; Huntley, B.; Wiltshire, A.; Hill, M.O.; Baxter, R. Slope, aspect and climate: Spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecol. Model. 2008, 216, 47–59. [Google Scholar] [CrossRef]




| Country | Farm | Pulp (n) | Peel (n) | Altitude (m) | Monthly Mean Temperature (°C) | Annual Rainfall (mm/Year) | Production System |
|---|---|---|---|---|---|---|---|
| Colombia | CO1 | 12 | 6 | 66 | 23.2 | 1837 | Conventional |
| Costa Rica | CR1 | 12 | 6 | 726 | 23.4 | 2857 | Conventional |
| CR2 | 12 | 6 | 47 | 24.4 | 5014 | Conventional | |
| CR3 | 12 | 6 | 24 | 26.3 | 1837 | Conventional | |
| Dominica Republic | DR1 | 12 | 6 | 65 | 26.7 | 925 | Organic |
| DR2 | 12 | 6 | 27 | 26.7 | 925 | Organic | |
| Ecuador | EC1 | 12 | 6 | 32 | 22.9 | 1511 | Organic |
| EC2 | 12 | 6 | 22 | 26.5 | 843 | Conventional | |
| Panama | PA1 | 12 | 6 | 20 | 19.7 | 3679 | Conventional |
| Peru | PE1 | 12 | 6 | 40 | 24.1 | 200 | Organic |
| Farm | δ13C | δ15N | δ18O | Al | Ba | Cu | Fe | Mn | Mo | Ni | Rb | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO1 | −23.0 ab ± 0.8 | 2.9 b ± 0.8 | 30.9 bc ± 0.5 | 0.8 bcd ± 0.2 | 1.6 a ± 0.2 | 3.6 cd ± 0.5 | 11.5 b ± 1.3 | 15.7 b ± 3.8 | 0.1 cd ± 0.1 | 0.3 a ± 0.1 | 1.9 c ± 0.3 | 2.6 b ± 0.4 | 6.1 bcd ± 1.1 |
| CR1 | −23.3 ac ± 0.5 | −0.9 f ± 0.9 | 31.9 a ± 0.7 | 0.9 ac ± 0.3 | 0.5 b ± 0.1 | 3.9 bcd ± 0.6 | 10.7 b ± 0.9 | 21.9 a ± 5.5 | 0.0 d ± 0.0 | 0.3 a ± 0.1 | 1.3 c ± 0.3 | 2.0 bc ± 0.5 | 5.2 de ± 0.8 |
| CR2 | −23.8 ac ± 0.6 | 1.1 cd ± 1.5 | 31.4 ab ± 0.6 | 1.2 a ± 0.5 | 1.6 a ± 0.3 | 4.5 ab ± 0.4 | 12.7 a ± 0.5 | 12.9 bc ± 1.9 | 0.2 b ± 0.0 | 0.1 cd ± 0.0 | 9.0 b ± 1.1 | 2.5 b ± 0.6 | 5.9 bce ± 1.2 |
| CR3 | −23.5 bc ± 0.4 | −0.5 ef ± 0.6 | 31.2 ac ± 0.4 | 0.9 bc ± 0.1 | 0.4 bc ± 0.0 | 3.7 cd ± 0.2 | 10.7 b ± 0.6 | 9.2 ce ± 2.9 | 0.2 b ± 0.1 | 0.2 cd ± 0.0 | 7.0 b ± 2.0 | 1.9 bc ± 0.2 | 5.9 bce ± 0.5 |
| DR1 | −23.3 ac ± 0.5 | 1.9 cd ± 0.4 | 30.5 c ± 0.3 | 0.6 cd ± 0.2 | 0.3 cd ± 0.0 | 4.2 ac ± 0.7 | 8.8 c ± 0.7 | 5.2 f ± 1.7 | 0.3 a ± 0.1 | 0.1 cd ± 0.0 | 1.9 c ± 0.8 | 3.3 a ± 0.6 | 7.0 ab ± 0.6 |
| DR2 | −23.2 ac ± 0.6 | 6.3 a ± 1.3 | 30.9 bc ± 1.1 | 0.6 cd ± 0.3 | 0.5 bc ± 0.2 | 4.5 bd ± 0.8 | 9.3 c ± 0.9 | 7.6 ef ± 2.5 | 0.2 b ± 0.1 | 0.1 d ± 0.0 | 2.9 c ± 1.8 | 3.5 a ± 0.6 | 7.1 a ± 1.1 |
| EC1 | −23.1 ac ± 0.6 | 1.5 ce ± 0.6 | 29.1 d ± 0.7 | 0.7 cd ± 0.2 | 0.2 d ± 0.1 | 2.8 e ± 0.2 | 8.6 c ± 0.5 | 5.6 ef ± 0.8 | 0.1 d ± 0.0 | 0.2 bc ± 0.0 | 7.3 b ± 2.6 | 1.2 de ± 0.4 | 6.0 bce ± 0.4 |
| EC2 | −23.6 bc ± 0.2 | −0.4ef ± 0.2 | 28.4 d ± 0.5 | 0.4 d ± 0.1 | 0.3 bd ± 0.0 | 3.6 cd ± 0.4 | 8.8 c ± 0.5 | 5.3 ef ± 1.2 | 0.2 bc ± 0.0 | 0.2 b ± 0.0 | 1.8 c ± 0.4 | 1.0 e ± 0.1 | 6.0 bcd ± 0.3 |
| PA1 | −23.4 bc ± 0.2 | 0.0 def ± 0.1 | 31.0 ac ± 0.4 | 0.8 bc ± 0.2 | 0.5 bd ± 0.1 | 4.7 a ± 0.4 | 12.7 a ± 0.8 | 11.6 cd ± 4.2 | 0.2 b ± 0.0 | 0.1 d ± 0.0 | 3.4 c ± 1.5 | 2.0 bc ± 0.4 | 6.8 ac ± 0.5 |
| PE1 | −22.6 a ± 0.3 | 1.7 c ± 0.3 | 31.5 ab ± 0.8 | 1.1 ab ± 0.2 | 0.4 bc ± 0.1 | 3.4 de ± 0.2 | 8.7 c ± 0.7 | 8.0 def ± 2.0 | 0.2 bc ± 0.0 | 0.1 d ± 0.0 | 12.1 a ± 5.2 | 1.7 cd ± 0.2 | 5.0 e ± 0.3 |
| Farm | δ13C | δ15N | δ18O | Al | Ba | Cr | Cu | Fe | Mn | Mo | Ni | Rb | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO1 | −25.0 bd ± 0.8 | 3.8 b ± 0.3 | 25.7 cd ± 0.3 | 24.3 bc ± 2.6 | 8.7 b ± 1.1 | 0.1 ab ± 0.0 | 5.1 ab ± 0.6 | 24.8 a ± 9.3 | 43.2 bcd ± 7.8 | 0.2 bc ± 0.1 | 0.4 a ± 0.1 | 3.1 d ± 0.7 | 22.0 c ± 3.5 | 17.8 ab ± 1.7 |
| CR1 | −24.7 bd ± 0.5 | −1.1 f ± 0.5 | 28.0 b ± 1.0 | 23.5 cd ± 7.5 | 3.6 cd ± 0.6 | 0.1 ab ± 0.1 | 4.0 cd ± 0.3 | 19.6 ac ± 1.4 | 82.2 a ± 15.6 | 0.1 d ± 0.0 | 0.3 ab ± 0.1 | 6.0 d ± 4.7 | 20.8 cd ± 3.1 | 17.8 ab ± 2.0 |
| CR2 | −25.0 bd ± 0.3 | 0.5 df ± 0.3 | 27.9 b ± 1.3 | 24.7 bc ± 5.9 | 10.6 a ± 1.5 | 0.1 ab ± 0.0 | 5.8 a ± 0.7 | 21.8 ab ± 1.2 | 61.7 ab ± 26.9 | 0.2 bc ± 0.0 | 0.2 b ± 0.0 | 24.3 bc ± 7.2 | 22.3 c ± 8.5 | 15.7 ab ± 1.9 |
| CR3 | −24.6 bc ± 0.3 | −0.3 ef ± 0.2 | 28.2 b ± 0.9 | 29.1 bc ± 13.6 | 3.4 cd ± 1.4 | 0.0 b ± 0.0 | 4.5 bc ± 0.8 | 16.2 bcd ± 4.7 | 35.0 ce ± 11.3 | 0.2 bc ± 0.0 | 0.2 c ± 0.1 | 16.4 bd ± 3.9 | 21.8 c ± 4.4 | 16.5 ab ± 1.6 |
| DR1 | −23.6 a ± 0.5 | 2.6 c ± 0.4 | 29.8 a ± 0.3 | 23.5 cd ± 4.8 | 2.4 cd ± 0.3 | 0.1 ab ± 0.0 | 3.7 cd ± 0.6 | 19.9 ac ± 2.5 | 18.0 ce ± 6.9 | 0.5 a ± 0.1 | 0.2 c ± 0.0 | 8.8 cd ± 5.3 | 41.5 a ± 4.6 | 19.0 a ± 2.5 |
| DR2 | −25.6 d ± 0.5 | 8.0 a ± 0.4 | 27.2 bc ± 1.3 | 5.4 e ± 1.4 | 3.0 cd ± 0.1 | 0.1 a ± 0.0 | 3.4 d ± 0.4 | 20.5 ac ± 2.4 | 31.7 ce ± 2.5 | 0.3 b ± 0.0 | 0.2 c ± 0.0 | 11.8 cd ± 5.3 | 33.7 b ± 1.9 | 15.3 ab ± 2.7 |
| EC1 | −24.4 ab ± 0.5 | 1.5 cde ± 0.5 | 26.7 bd ± 0.7 | 11.1 de ± 2.3 | 1.8 d ± 0.4 | 0.1 ab ± 0.0 | 3.6 cd ± 0.4 | 16.5 bcd ± 1.0 | 28.0 ce ± 3.6 | 0.1 cd ± 0.0 | 0.3 bc ± 0.0 | 28.7 b ± 5.2 | 13.3 de ± 2.4 | 15.5 ab ± 1.4 |
| EC2 | −24.9 bd ± 0.3 | 0.5 df ± 0.2 | 25.2 d ± 0.5 | 21.3 cd ± 4.3 | 2.1 cd ± 0.1 | 0.1 ab ± 0.0 | 4.2 bd ± 0.3 | 16.3 bcd ± 1.4 | 21.2 de ± 7.0 | 0.2 bc ± 0.0 | 0.3 bc ± 0.1 | 10.8 cd ± 7.6 | 10.5 e ± 2.1 | 14.3 b ± 1.2 |
| PA1 | −25.3 cd ± 0.3 | 0.8 cde ± 0.6 | 28.4 ab ± 0.8 | 19.8 cd ± 1.7 | 2.6 cd ± 0.6 | 0.0 b ± 0.0 | 5.6 a ± 0.5 | 13.7 cd ± 0.8 | 45.8 bc ± 18.7 | 0.2 bc ± 0.0 | n.d. | 11.9 cd ± 6.0 | 23.5 c ± 3.8 | 19.0 a ± 1.9 |
| PE1 | −23.6 a ± 0.2 | 1.9 cd ± 0.5 | 27.4 b ± 0.8 | 37.3 b ± 11.7 | 2.9 cd ± 0.2 | 0.1 ab ± 0.0 | 4.4 bc ± 0.5 | 12.2 d ± 1.2 | 34.0 ce ± 4.5 | 0.2 b ± 0.0 | 0.2 c ± 0.1 | 47.7 a ± 20.5 | 17.0 ce ± 1.8 | 18.2 a ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Erasmus, S.W.; van Ruth, S.M. Preliminary Study on Tracing the Origin and Exploring the Relations between Growing Conditions and Isotopic and Elemental Fingerprints of Organic and Conventional Cavendish Bananas (Musa spp.). Foods 2021, 10, 1021. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10051021
Wang Z, Erasmus SW, van Ruth SM. Preliminary Study on Tracing the Origin and Exploring the Relations between Growing Conditions and Isotopic and Elemental Fingerprints of Organic and Conventional Cavendish Bananas (Musa spp.). Foods. 2021; 10(5):1021. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10051021
Chicago/Turabian StyleWang, Zhijun, Sara W. Erasmus, and Saskia M. van Ruth. 2021. "Preliminary Study on Tracing the Origin and Exploring the Relations between Growing Conditions and Isotopic and Elemental Fingerprints of Organic and Conventional Cavendish Bananas (Musa spp.)" Foods 10, no. 5: 1021. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10051021