Effects of Konjac Glucomannan on Oil Absorption and Safety Hazard Factor Formation of Fried Battered Fish Nuggets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
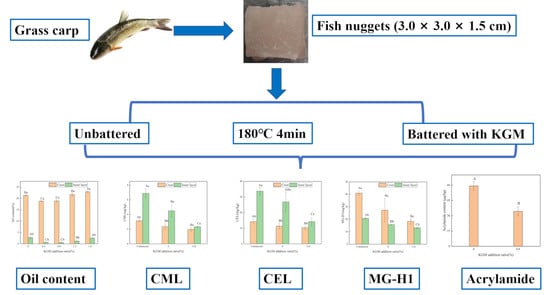
2.2. Preparation of Fried Battered Fish Nuggets
2.3. Measurement of Moisture and Oil Contents
2.4. Measurement of Surface Oil, Penetrated Surface Oil and Structure Oil Contents
2.5. Measurement of Battering Rate
2.6. Measurement of Malondialdehyde Content
2.7. Microstructure Observation and Calculation of Pore Equivalent Diameter
2.8. Measurement of Nonfluorescent AGEs
2.9. Measurement of Fluorescent AGEs
2.10. Measurement of Acrylamide Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Moisture and Oil Contents of Fried Battered Fish Nuggets
3.2. Battering Rate of Fried Battered Fish Nuggets
3.3. Oil Distribution of Fried Battered Fish Nuggets
3.4. Microstructures of the Crust of Fried Battered Fish Nuggets
3.5. Malondialdehyde Content of Fried Battered Fish Nuggets
3.6. AGEs of Fried Fish Nuggets
3.7. Acrylamide Content of Fried Battered Fish Nuggets
3.8. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trujillo-Agudelo, S.; Osorio, A.L.; Gómez, F. Evaluation of the application of an edible coating and different frying temperatures on acrylamide and fat content in potato chips. J. Food Process Eng. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Wu, R.L.; Liu, M.M.; Qin, R.K. Effect of air frying conditions on the quality of fish paste/pork composite gel. J. Huazhong Agric. Univ. 2020, 39, 59–66. [Google Scholar]
- Martínez-Pineda, M.; Yagüe-Ruiz, C.; Vercet, A. How batter formulation can modify fried tempura-battered zucchini chemical and sensory characteristics? Foods 2020, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Brannan, R.G.; Pettit, K. Reducing the oil content in coated and deep-fried chicken using whey protein. Lipid Technol. 2015, 27, 131–133. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Overview on mitigation of acrylamide in starchy fried and baked foods. J. Sci. Food Agric. 2018, 98, 4385–4394. [Google Scholar] [CrossRef]
- Kavousi, P.; Mirhosseini, H.; Ghazali, H. Formation and reduction of 5-hydroxymethylfurfural at frying temperature in model system as a function of amino acid and sugar composition. Food Chem. 2015, 182, 164–170. [Google Scholar] [CrossRef]
- Dourado, C.; Pinto, C.; Barba, F.J. Innovative non-thermal technologies affecting potato tuber and fried potato quality. Trends Food Sci. Technol. 2019, 88, 274–289. [Google Scholar] [CrossRef]
- Jacob, T.L.; Jalal, D.; Michael, O.N. Effective strategies for reduction of oil content in deep-fat fried foods: A review. Trends Food Sci. Technol. 2019, 92, 172–183. [Google Scholar]
- Barbut, S. Frying-Effect of coating on crust microstructure, color, and texture of lean meat portions. Meat Sci. 2013, 93, 269–274. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Zhang, J. Effect of pretreatment methods on acrylamide content in fried potato products. Food Sci. Technol. 2021, 46, 189–193. [Google Scholar]
- Chen, S.D.; Chen, H.H.; Chao, Y.C. Effect of batter formula on qualities of deep-fat and microwave fried fish nuggets. J. Food Eng. 2009, 95, 359–364. [Google Scholar] [CrossRef]
- Brannan, R.G.; Mah, E.; Schott, M. Influence of ingredients that reduce oil absorption during immersion frying of battered and breaded foods. Eur. J. Lipid Sci. Technol. 2014, 116, 240–254. [Google Scholar] [CrossRef]
- Lumanlan, J.C.; Fernando, W.M.A.D.B.; Jayasena, V. Mechanisms of oil uptake during deep frying and applications of predrying and hydrocolloids in reducing fat content of chips. Int. J. Food Sci. Technol. 2020, 55, 1661–1670. [Google Scholar] [CrossRef]
- Xie, D.; Chen, J.W.; Zeng, H. Effect of hydrophilic colloid on the fat content and quality of fried battered and breaded fish nuggets. Food Sci. 2016, 37, 45–50. [Google Scholar]
- Jiang, Y.; Qin, R.K.; Jia, C.H. Hydrocolloid effects on Nε-carboxymethyllysine and acrylamide of deep-fried fish nuggets. Food Biosci. 2020, 39, 100797. [Google Scholar] [CrossRef]
- Meng, F.D.; Liu, D.Y.; Li, Y.C. Research progress of the structures, properties and modifications of Konjac glucomannan. Sci. Technol. Food Ind. 2016, 37, 394–399. [Google Scholar]
- Fu, Y.Y.; Mei, C.; Wang, Y.P. Recent advances of edible coatings in reservation of fruits and vegetables. Xinjiang Agric. Sci. 2019, 56, 2263–2274. [Google Scholar]
- Supawong, S.; Park, J.W.; Thawornchinsombut, S. Fat blocking roles of fish proteins in fried fish cake. LWT Food Sci. Technol. 2018, 97, 462–468. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA, 2004. [Google Scholar]
- Bouchon, P.; Aguilera, J.M.; Pyle, D.L. Structure oil-absorption relationships during deep-fat frying. J. Food Sci. 2003, 68, 2711–2716. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.; Zhai, J. Reduction of the fat content of battered and breaded fish balls during deep-fat frying using fermented bamboo shoot dietary fiber. LWT Food Sci. Technol. 2016, 73, 425–431. [Google Scholar] [CrossRef]
- Zhu, Y.A.; Liu, Y.M.; Zhang, Q.L. Effect of Heating Methods on Gel Properties of Silver Carp Fish Surimi. Food Sci. 2011, 32, 107–110. [Google Scholar]
- Niquet-Léridon, C.; Tessier, F.J. Quantification of Nε-carboxymethyl-lysine in selected chocolate-flavored drink mixes using high-performance liquid chromatography-linear ion trap tandem mass spectrometry. Food Chem. 2011, 126, 655–663. [Google Scholar] [CrossRef]
- Michalak, J.; Czarnowska-Kujawska, M.; Gujska, E. Acrylamide and Thermal Processing Indexes in Market-Purchased Food. Int. J. Environ. Res. Public Health 2019, 16, 4724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zeng, L.; Wang, X. The influence of Konjac glucomannan on the functional and structural properties of wheat starch. Food Sci. Nutr. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Rahimi, J.; Ngadi, M.O. Surface ruptures of fried batters as influenced by batter formulations. J. Food Eng. 2015, 152, 50–56. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Adv. Colloid Interface Sci. 2006, 128, 267–272. [Google Scholar] [CrossRef]
- Wei, E.H.; Wu, J.H.; Liu, B. Characterization and Application of Konjac Glucomannan in Food Industry. Food Ind. 2016, 37, 239–241. [Google Scholar]
- Garmakhany, A.D.; Mirzaei, H.O.; Nejad, M.K. Study of oil uptake and some quality attributes of potato chips affected by hydrocolloids. Eur. J. Lipid Sci. Technol. 2008, 110, 1045–1049. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.T.; Fan, D.M. The description of oil absorption behavior of potato chips during the frying. LWT Food Sci. Technol. 2018, 96, 119–126. [Google Scholar] [CrossRef]
- Tavares, W.P.S.; Dong, S.Y.; Jin, W.Y. Effect of different cooking conditions on the profiles of Maillard reaction products and nutrient composition of hairtail (Thichiurus lepturus) fillets. Food Res. Int. 2018, 103, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Cai, W.; Peppa, M. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Zhou, Q.; Fan, D. Novel roles of hydrocolloids in foods: Inhibition of toxic maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Chen, G.J.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 168, 190–195. [Google Scholar] [CrossRef]
- Shan, J.; Chen, J.; Xie, D. Effect of xanthan gum/soybean fiber ratio in the batter on oil absorption and quality attributes of fried breaded fish nuggets. J. Food Sci. 2018, 83, 1832–1838. [Google Scholar] [CrossRef]
- Zhu, Z.S.; Huang, M.; Cheng, Y.Q. A comprehensive review of Nε-carboxymethyllysine and Nε-carboxyethyllysine in thermal processed meat products. Trends Food Sci. Technol. 2020, 98, 30–40. [Google Scholar] [CrossRef]
- Ma, X.J.; Xiong, J. Nutrition evaluation of protein in light liquid phase produced during the process of wheat starch production by decanter centrifuge. Sci. Technol. Food Ind. 2011, 32, 83–86. [Google Scholar]
- Li, Z.Y.; Ding, H.X.; Zhang, L. Comparative analysis on the nutritional quality of grass carp muscle from different habitats. Food Ferment. Ind. 2021, 47, 133–139. [Google Scholar]
- Trevisan, A.J.B.; Lima, D.A.; Sampaio, G.R. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016, 196, 161–169. [Google Scholar] [CrossRef]
- Granvogl, M.; Jezussek, M.; Koehler, P. Quantitation of 3-aminopropionamide in potatoes-A minor but potent precursor in acrylamide formation. J. Agric. Food Chem. 2004, 52, 4751–4757. [Google Scholar] [CrossRef]
- Becalski, A.; Lau, B.P.; Lewis, D. Acrylamide in foods: Occurrence, sources, and modeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef]
- Wang, P.P.; Zhu, Y.C.; Liu, Y.B. Research Process of Effect of Lipid on Acrylamide Formation During Frying and Baking. J. Chin. Cereals Oils Assoc. 2017, 32, 140–146. [Google Scholar]
- Kobayashi, A.; Gomikawa, S.; Yamazaki, A. Elimination of Acrylamide by Moderate Heat Treatment below 120 °C with Lysine and Cysteine. Food Sci. Technol. Res. 2014, 20, 979–985. [Google Scholar] [CrossRef] [Green Version]





| KGM Addition Ratio (%) | Wheat Flour (%) | Modified Starch (%) | Baking Powder (%) | Salt (%) | KGM (%) |
|---|---|---|---|---|---|
| 0 | 58 | 40 | 0.5 | 1.5 | 0 |
| 0.4 | 57.6 | 40 | 0.5 | 1.5 | 0.4 |
| 0.8 | 57.2 | 40 | 0.5 | 1.5 | 0.8 |
| 1.2 | 56.8 | 40 | 0.5 | 1.5 | 1.2 |
| 1.6 | 56.4 | 40 | 0.5 | 1.5 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wu, R.; Hu, B.; Jia, C.; Rong, J.; Xiong, S.; Liu, R. Effects of Konjac Glucomannan on Oil Absorption and Safety Hazard Factor Formation of Fried Battered Fish Nuggets. Foods 2022, 11, 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101437
Sun J, Wu R, Hu B, Jia C, Rong J, Xiong S, Liu R. Effects of Konjac Glucomannan on Oil Absorption and Safety Hazard Factor Formation of Fried Battered Fish Nuggets. Foods. 2022; 11(10):1437. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101437
Chicago/Turabian StyleSun, Jingwen, Runlin Wu, Benlun Hu, Caihua Jia, Jianhua Rong, Shanbai Xiong, and Ru Liu. 2022. "Effects of Konjac Glucomannan on Oil Absorption and Safety Hazard Factor Formation of Fried Battered Fish Nuggets" Foods 11, no. 10: 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101437