Modeling Conjugate Heat Transfer in an Anode Baking Furnace Using OpenFoam
Abstract
:1. Introduction
2. Heating Section of Anode Baking Furnace
2.1. Computational Domain for Flue and Lining
2.2. Computational Mesh in Flue and Lining Domain
3. Modeling Non-Premixed Turbulent Combustion and Conjugate Heat Transfer in OpenFoam
3.1. Flow of Mixture of Gasses in the Flue
3.1.1. Conservation of Mass of the Mixture
3.1.2. Conservation of Momentum of the Mixture
3.1.3. Conservation of Energy of the Mixture
3.1.4. Conservation of the Chemical Species
3.1.5. Computation of the Radiative Heat Flux
3.2. Heat Transfer in the Refractory Lining
3.3. Zeldovich Thermal Nitric-Oxide Post-Processing
3.4. Implementation in OpenFoam
- step (1/3): in the first step, we allow the non-reactive flow field to fully develop in the flue from s to s. The patch separating the flue and the lining is set to be thermally insulating. We run as solver reactingFoam with psiThermo as thermodynamics, the transonic option set to false (allowing density variations in the pressure equation to be neglected), both the combustion and radiation switched off and with a fixed time step equal to ;
- step (2/3): in the second step, we allow the reactive flow field, the chemical species concentration and the incident radiation to fully develop in the flue from s to s. The patch separating the flue and the lining is again set to be thermally insulating. We run as solver reactingFoam with psiThermo as thermodynamics and the transonic option set to false as before. This time we switch on both the combustion and the radiative heat transfer. We use a fixed time step equal to . At s we run the NOxFoam post-processor to compute the nitric-oxide concentration in the adiabatic case as a reference
- step (3/3): in the third step, we permit the heat generated in the flue in the adiabatic case to be transported to the lining. We allow all fields in the flue and the lining to fully develop from s to s. We run as solver multiregionReactingFoam with psiThermo as thermodynamics, the transonic option set to false, and both the combustion and the radiative heat transfer switched on. We use a variable time step restricted by a Courant-Friedricks-Lev number equal to . In the final iterations a time step is equal to , i.e., ten times smaller than in the previous two simulation steps. We attribute this reduction of the time-step to the thermal stiffness of the lining. At s we run the NOxFoam post-processor to compute the nitric-oxide concentration in the non-adiabatic case.
4. Numerical Results
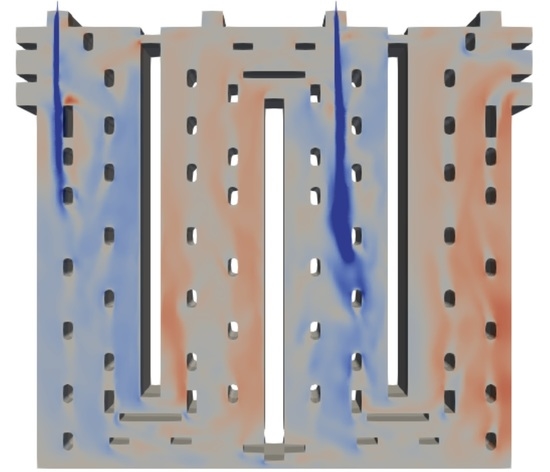
4.1. Computed Fluid Flow
4.2. Computed Temperature
4.3. Computed Equivalence Ratio
4.4. Computed Mass Fraction of Fuel, Oxidizer and Combustion Products
4.5. Computed Thermal Absorption Coefficient
4.6. Computed OH and Thermal NO Mass Fraction
4.7. Model Validation Using In-Situ Measurements at the Factory
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbol | Meaning | Symbol | Meaning |
| Gas density, kg/m | Optical Thickness, m | ||
| Refractory density, kg/m | Turbulent time scale, s | ||
| u | Velocity, m/s | Diffusion time scale, s | |
| s | Velocity Deformation Tensor, 1/s | Specific heat capacity, J/kg·C | |
| p | Reference press, Pa | T | Gas temperature, C |
| Molecular viscosity, kg/m·s | Refractory temperature, C | ||
| Gas thermal conductivity, W/m·K | Wall Temperature, C | ||
| Refractory thermal conductivity, W/m·K | Boltzmann constant, W/m·K | ||
| Turbulent viscosity, kg/m·s | Chemical heat source, J/m | ||
| Turbulent kinematic viscosity, m/s | Radiative heat source, J/m | ||
| k | Turbulent kinetic energy, m/s | Wall emissivity | |
| Turbulent dissipation rate, m/s | Molecular Mass Diffusivity, m/s | ||
| Specific enthalpy, J | Radiative heat flux, J/m·s | ||
| Mass fraction | G | Total Incident radiation, W/m | |
| Chemical source term, kg/(m·s) | Absorption coefficient, 1/m |
References
- Grégoire, F.; Gosselin, L. Comparison of three combustion models for simulating anode baking furnaces. Int. J. Therm. Sci. 2018, 129, 532–544. [Google Scholar] [CrossRef]
- Tajik, A.R.; Shamim, T.; Ghoniem, A.F.; Abu Al-Rub, R.K. The Impact of Critical Operational Parameters on the Performance of the Aluminum Anode Baking Furnace. J. Energy Resour. Technol. Trans. ASME 2021, 143, 062103. [Google Scholar] [CrossRef]
- Oumarou, N.; Kocaefe, D.; Kocaefe, Y.; Morais, B.; Chabot, J. A dynamic process model for simulating horizontal anode baking furnaces. Mater. Sci. Technol. Conf. Exhib. 2013, 3, 2017. [Google Scholar]
- Severo, D.S.; Gusberti, V.; Pinto, E.C.V. Advanced 3D modelling for anode baking furnaces. Light Met. 2005, 697–702. [Google Scholar]
- Tajik, A.R.; Shamim, T.; Zaidani, M.; Abu Al-Rub, R.K. The effects of flue-wall design modifications on combustion and flow characteristics of an aluminum anode baking furnace-CFD modeling. Appl. Energy 2018, 230, 207–219. [Google Scholar] [CrossRef]
- Zaidani, M.; Tajik, A.R.; Qureshi, Z.A.; Shamim, T.; Abu Al-Rub, R.K. Investigating the flue-wall deformation effects on performance characteristics of an open-top aluminum anode baking furnace. Appl. Energy 2018, 231, 1033–1049. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, C.; Xu, M. Simulating the heat transfer process of horizontal anode baking furnace. Dev. Chem. Eng. Miner. Process. 2004, 12, 427–440. [Google Scholar] [CrossRef]
- Lahaye, D.; el Abbassi, M.; Vuik, C.; Talice, M.; Juretić, F. Mitigating Thermal NOx by Changing the Secondary Air Injection Channel: A Case Study in the Cement Industry. Fluids 2020, 5, 220. [Google Scholar] [CrossRef]
- Nakate, P.; Lahaye, D.; Vuik, C.; Talice, M. Analysis of the aerodynamics in the heating section of an anode baking furnace using non-linear finite element simulations. Fluids 2021, 6, 46. [Google Scholar] [CrossRef]
- Nakate, P.; Lahaye, D.; Vuik, C. The Nitric Oxide Formation in Anode Baking Furnace through Numerical Modeling. Int. J. Thermofluids 2021, 12, 100–122. [Google Scholar] [CrossRef]
- Juretic, F. cfMesh Version 1.1 Users Guide. Available online: http://cfmesh.com/wp-content/uploads/2015/09/User_Guide-cfMesh_v1.1.pdf (accessed on 1 November 2020).
- Lahaye, D.; Nakate, P.; Vuik, C.; Talice, M.; Juretić, F. Turbulent Non-Premixed Combustion in Single Heating Section of an Anode Baking Ring Furnace; OpenFoam Workshop: Dublin, Ireland, 2021. [Google Scholar]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A Tensorial Approach to Computational Continuum Mechanics Using Object-oriented Techniques. Comput. Phys. 1998, 12, 620–631. [Google Scholar] [CrossRef]
- Peeters, T. Numerical Modeling of Turbulent Natural-Gas Diffusion Flames. Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 1995. [Google Scholar]
- Lewandowski, M.T.; Parente, A.; Pozorski, J. Generalised Eddy Dissipation Concept for MILD combustion regime at low local Reynolds and Damkohler numbers. Part 1: Model framework development. Fuel 2020, 278, 117743. [Google Scholar] [CrossRef]
- Gupta, H.; Teerling, O.J.; van Oijen, J.A. Effect of progress variable definition on the mass burning rate of premixed laminar flames predicted by the Flamelet Generated Manifold method. Combust. Theory Model. 2021, 25, 631–645. [Google Scholar] [CrossRef]
- Cuenot, B.; Shum-Kivan, F.; Blanchard, S. The thickened flame approach for non-premixed combustion: Principles and implications for turbulent combustion modeling. Combust. Flame 2021, 2021, 111702. [Google Scholar] [CrossRef]
- Gaikwad, P.; Sreedhara, S. OpenFOAM based conditional moment closure (CMC) model for solving non-premixed turbulent combustion: Integration and validation. Comput. Fluids 2019, 190, 362–373. [Google Scholar] [CrossRef]
- Zhang, C.; Bounds, C.P.; Foster, L.; Uddin, M. Turbulence Modeling Effects on the CFD Predictions of Flow over a Detailed Full-Scale Sedan Vehicle. Fluids 2019, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Suarez, J.A.G.; Mejia, A.G.; Uruena, C.H.G. Low-Cost Eddy-Resolving Simulation in the Near-Field of an Annular Swirling Jet for Spray Drying Applications. ChemEngineering 2021, 5, 80. [Google Scholar] [CrossRef]
- Huang, X.; Tummers, M.J.; van Veen, E.H.; Roekaerts, D.J.E.M. Modelling of MILD combustion in a lab-scale furnace with an extended FGM model including turbulence-radiation interaction. Combust. Flame 2022, 237, 111634. [Google Scholar] [CrossRef]
- Modest, M.F.; Haworth, D.C. Radiative Heat Transfer in Turbulent Combustion Systems: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Baukal, C. Industrial Combustion Pollution and Control; Environmental Science & Pollution; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Poinsot, T.; Veynante, D. Theoretical and Numerical Combustion, 2nd ed.; R.T. Edwards, Inc.: Philadelphia, PA, USA, 2005. [Google Scholar]
- Dorfman, A.S. Conjugate Problems in Convective Heat Transfer; Heat Transfer Series; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Magnussen, B.F.; Hjertager, B.H. On mathematical modeling of turbulent combustion with special emphasis on soot formation and combustion. Symp. (Int.) Combust. 1977, 16, 719–729. [Google Scholar] [CrossRef]
- Versteeg, H.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method, 2nd ed.; Pearson Education Limited: London, UK, 2007. [Google Scholar]
- Kadar, A.H. Modelling Turbulent Non-Premixed Combustion in Industrial Furnaces. Master’s Thesis, Technical University of Delft, Delft, The Netherlands, 2015. [Google Scholar]
- Abou-Taouk, A.; Farcy, B.; Domingo, P.; Vervisch, L.; Sadasivuni, S.; Eriksson, L.-E. Optimized reduced chemistry and molecular transport for large eddy simulation of partially premixed combustion in a gas turbine. Combust. Sci. Technol. 2016, 188, 21–39. [Google Scholar] [CrossRef]











| Properties of the Lining | |
|---|---|
| thickness (D) | 0.11 m |
| density () | |
| specific heat capacity () | |
| thermal conductivity (k) | |
| wall emissivity () | |
| outside wall temperature () | |
| Inlet Conditions of Preheated Air | |
|---|---|
| x-Component of velocity () | |
| Temperature (T) | |
| Turbulent kinetic energy (k) | |
| Turbulent dissipation () | |
| Inlet Conditions of Natural Gas at Both Burners | |
| y-Component of velocity () | |
| Temperature (T) | |
| Turbulent kinetic energy (k) | |
| Turbulent dissipation () | |
| Zone | Location in the Geometry | Smallest Edge Length |
|---|---|---|
| Zone 1 | Interior of Two Fuel Pipes | 2 mm |
| Zone 2 | Near Walls of Tie Bricks with Curved Corners | 8 mm |
| Zone 3 | Inside of Refinement Zone at Two Fuel Pipe Exits | 16 mm |
| Zone 4 | Near Walls of Tie Bricks with Sharp Corners | 32 mm |
| Zone 5 | Near Walls of Main Channel | 32 mm |
| Zone 6 | Interior of Main Channel | 64 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahaye, D.; Nakate, P.; Vuik, K.; Juretić, F.; Talice, M. Modeling Conjugate Heat Transfer in an Anode Baking Furnace Using OpenFoam. Fluids 2022, 7, 124. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids7040124
Lahaye D, Nakate P, Vuik K, Juretić F, Talice M. Modeling Conjugate Heat Transfer in an Anode Baking Furnace Using OpenFoam. Fluids. 2022; 7(4):124. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids7040124
Chicago/Turabian StyleLahaye, Domenico, Prajakta Nakate, Kees Vuik, Franjo Juretić, and Marco Talice. 2022. "Modeling Conjugate Heat Transfer in an Anode Baking Furnace Using OpenFoam" Fluids 7, no. 4: 124. https://0-doi-org.brum.beds.ac.uk/10.3390/fluids7040124