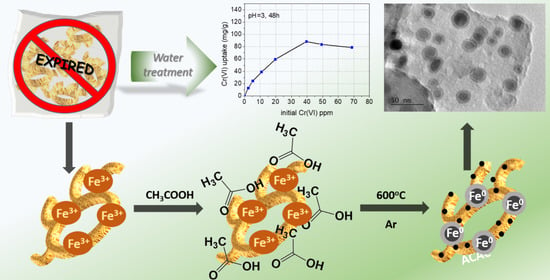
Use of a Hybrid Porous Carbon Material Derived from Expired Polysaccharides Snack/Iron Salt Exhibiting Magnetic Properties, for Hexavalent Chromium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Synthesis of Materials
2.2.1. Synthesis of AC-Snack
2.2.2. Synthesis of mAC-Snack
2.3. Characterization of Materials
2.4. Batch Experiments
2.5. Oxidation Experiments for the Determination of Cr3+ in the Solution
2.6. Thermodynamics of Cr(VI) Adsorption
3. Results and Discussion
3.1. Material’s Characterization
3.2. Hexavalent Chromium Removal
3.3. Thermodynamics of Cr(VI) Adsorption. Results for Gibbs Free Energy
3.4. Study of the Removal Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jjagwe, J.; Olupot, P.; Menya, E.; Kalibbala, H. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Uriburu-Gray, M.; Pinar-Serrano, A.; Cavus, G.; Knipping, E.; Aucher, C.; Conesa-Cabeza, A.; Satti, A.; Amantia, D.; Martínez-Crespiera, S. Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries. Nanomaterials 2020, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Benítez, A.; Amaro-Gahete, J.; Chien, Y.-C.; Caballero, Á.; Morales, J.; Brandell, D. Recent advances in lithium-sulfur batteries using biomass-derived carbons as sulfur host. Renew. Sustain. Energy Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.; Chen, W. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review. Sep. Purif. Technol. 2020, 253, 117536. [Google Scholar] [CrossRef]
- Secondi, L. Expiry Dates, Consumer Behavior, and Food Waste: How Would Italian Consumers React If There Were No Longer “Best Before” Labels? Sustainability 2019, 11, 6821. [Google Scholar] [CrossRef] [Green Version]
- Stenmarck, Â.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Scherhaufer, S. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F. Saccharide Characteristics and Their Potential Health Effects in Perspective. Front. Nutr. 2020, 7, 75. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefin. 2019, 9, 775–802. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Pariser, H.H.; Backeberg, N.R.; Masson, O.C.M.; Bedder, J.C.M. Changing nickel and chromium stainless steel markets-A review. J. South. Afr. Inst. Min. Metall. 2018, 118, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.K. Hexavalent chromium [Cr(VI)] removal by acid modified waste activated carbons. J. Hazard. Mater. 2009, 171, 116–122. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Aroua, M.K.; Zuki, F.M.; Sulaiman, N.M. Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. J. Hazard. Mater. 2007, 147, 752–758. [Google Scholar] [CrossRef]
- Pehlivan, E.; Cetin, S. Sorption of Cr(VI) ions on two Lewatit-anion exchange resins and their quantitative determination using UV-visible spectrophotometer. J. Hazard. Mater. 2009, 163, 448–453. [Google Scholar] [CrossRef]
- Rai, M.K.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.S.; Rai, B.N. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour.-Effic. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Selvi, K.; Pattabhi, S.; Kadirvelu, K. Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 2001, 80, 87–89. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, J.; Mandal, B. Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J. Hazard. Mater. 2009, 168, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) sorption properties of activated carbon produced via pyrolysis of the “Posidonia oceanica” seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef]
- Ranganathan, K. Chromium removal by activated carbons prepared from Casurina equisetifolia leaves. Bioresour. Technol. 2000, 73, 99–103. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, S.N.; Rath, P. Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust. Biochem. Eng. J. 2006, 31, 216–222. [Google Scholar] [CrossRef]
- Dubey, S.P.; Gopal, K. Adsorption of chromium(VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghasemi, S.; Rustaiyan, A. Removal of Hexavalent Chromium Using Activated Carbons Derived From Marine Algae Gracilaria and Sargassum Sp. J. Mar. Sci. Technol. 2010, 18, 587–592. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous Activated Carbon Derived via Pyrolysis Process of Spent Coffee: Structural Characterization. Investigation of Its Use for Hexavalent Chromium Removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Bae, S.; Collins, R.N.; Waite, T.D.; Hanna, K. Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review. Environ. Sci. Technol. 2018, 52, 12010–12025. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.; Phull, A.; Sarfraz Ali, J.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Chen, D.; Zhang, H.; Wu, J.; He, F.; Wang, J.; Chen, J. Stability of hydrous ferric oxide nanoparticles encapsulated inside porous matrices: Effect of solution and matrix phase. Chem. Eng. J. 2018, 347, 870–876. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Wang, W.; Teng, W.; Zhang, W.-X. Stabilization of nanoscale zero-valent iron in water with mesoporous carbon (nZVI@MC). J. Environ. Sci. 2019, 81, 28–33. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, S.; Kaila, C.; Su, X.; Wu, J.; Karki, A.B.; Young, D.P.; Guo, Z. Carbon-stabilized iron nanoparticles for environmental remediation. Nanoscale 2010, 2, 917–919. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Fortunato, M.E.; Dastgheib, S.A.; Rostam-Abadi, M.; Rood, M.J.; Suslick, K.S. Synthesis and characterization of iron-impregnated porous carbon spheres prepared by ultrasonic spray pyrolysis. Carbon 2011, 49, 587–598. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Karakassides, A.; Baikousi, M.; Gioti, C.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Douvalis, A.P.; Salmas, C.E.; Karakassides, M.A. Nanoporous Carbon Magnetic Hybrid Derived from Waterlock Polymers and Its Application for Hexavalent Chromium Removal from Aqueous Solution. J. Carbon Res. 2021, 7, 69. [Google Scholar] [CrossRef]
- Baikousi, M.; Bourlinos, A.B.; Douvalis, A.; Bakas, T.; Anagnostopoulos, D.F.; Tuček, J.; Šafaářovaá, K.; Zbořil, R.; Karakassides, M.A. Synthesis and Characterization of γ-Fe2O3/Carbon Hybrids and Their Application in Removal of Hexavalent Chromium Ions from Aqueous Solutions. Langmuir 2012, 28, 3918–3930. [Google Scholar] [CrossRef]
- Douvalis, A.; Polymeros, A.; Bakas, T. IMSG09: A 57Fe119Sn Mössbauer spectra computer fitting program with novel interactive user interface. J. Phys. Conf. Ser. 2010, 217, 012014. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A New Model for Capillary Condensation−Evaporation Hysteresis Based on a Random Corrugated Pore Structure Concept: Prediction of Intrinsic Pore Size Distributions. 1. Model Formulation. Ind. Eng. Chem. Res. 2000, 39, 3747–3763. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A New Model for Capillary Condensation−Evaporation Hysteresis Based on a Random Corrugated Pore Structure Concept: Prediction of Intrinsic Pore Size Distribution. 2. Model Application. Ind. Eng. Chem. Res. 2000, 39, 3764–3777. [Google Scholar] [CrossRef]
- DOWNLOADS, CPSM_Nitrogen. Available online: http://users.uoi.gr/ksalmas/ (accessed on 20 June 2020).
- Salmas, C.E.; Androutsopoulos, G.P. Rigid Sphere Molecular Model Enables an Assessment of the Pore Curvature Effect upon Realistic Evaluations of Surface Areas of Mesoporous and Microporous Materials. Langmuir 2005, 21, 11146–11160. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, R.J.; Hyduke, D.R.; Lastoskie, C.M. Pore Size Analysis of Activated Carbons from Argon and Nitrogen Porosimetry Using Density Functional Theory. Langmuir 2000, 16, 5041–5050. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov Equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Salmas, C.E.; Ladavos, A.K.; Skaribas, S.P.; Pomonis, P.J.; Androutsopoulos, G.P. Evaluation of Microporosity, Pore Tortuosity, and Connectivity of Montmorillonite Solids Pillared with LaNiOx Binary Oxide. A Combined Application of the CPSM Model, the αs-Plot Method and a Pore Percolation−Connectivity Model. Langmuir 2003, 19, 8777–8786. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Abdullah, A.H.; Chalimah, S.; Primadona, I.; Hanantyo, M. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef] [Green Version]
- Feairheller, W.R.; Katon, J.E. The vibrational spectra of acrylic acid and sodium acrylate. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 2225–2232. [Google Scholar] [CrossRef]
- Grabowska, B.; Holtzer, M. Structural examination of the cross-linking reaction mechanism of polyacrylate binding agents. Arch. Metall. Mater. 2009, 54, 427–437. [Google Scholar]
- Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Characterization of activated carbon, graphitized carbon fibers and synthetic diamond powder using TPD and DRIFTS. Carbon 1998, 36, 1821–1831. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New starch-derived mesoporous carbonaceous materials with tunable properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Catalysts on Formation of Carbon-Encapsulated Iron Nanoparticles from Kraft Lignin. Materials 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Ennas, G.; Marongiu, G.; Musinu, A. Characterization of nanocrystalline γ–Fe2O3 prepared by wet chemical method. J. Mater. Res. 1998, 14, 1570. [Google Scholar] [CrossRef]
- Tongsri, R.; Vetayanugul, B. Thermal analysis of Fe-carbide and Fe-C mixtures. J. Met. Mater. Miner. 2010, 20, 45–49. [Google Scholar]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small 2020, 16, 1906198. [Google Scholar] [CrossRef]
- Defilippi, C.; Mukadam, M.O.; Nicolae, S.A.; Lees, M.R.; Giordano, C. Iron Carbide@Carbon Nanocomposites: A Tool Box of Functional Materials. Materials 2019, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Misof, B.M.; Roschger, P.; Fratzl, P. Imaging Mineralized Tissues in Vertebrates. Compr. Biomater. 2011, 3, 407–426. [Google Scholar] [CrossRef]
- Liu, D.-H.; Guo, Y.; Zhang, L.-H.; Li, W.-C.; Sun, T.; Lu, A.-H. Switchable Transport Strategy to Deposit Active Fe/Fe3C Cores into Hollow Microporous Carbons for Efficient Chromium Removal. Small 2013, 9, 3852–3857. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)–Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, S.; Jin, F.; Huang, J.; Bao, N. Characterization and mechanism analysis of activated carbon fiber felt-stabilized nanoscale zero-valent iron for the removal of Cr(VI) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 59–66. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Xu, C.-H.; Zhu, L.-J.; Wang, X.-H.; Lin, S.; Chen, Y.-M. Fast and Highly Efficient Removal of Chromate from Aqueous Solution Using Nanoscale Zero-Valent Iron/Activated Carbon (NZVI/AC). Water. Air. Soil Pollut. 2014, 225, 1845. [Google Scholar] [CrossRef]
- Cui, Y.; He, H.; Atkinson, J.D. Iron/Carbon Composites for Cr(VI) Removal Prepared from Harmful Algal Bloom Biomass via Metal Bioaccumulation or Biosorption. ACS Sustain. Chem. Eng. 2019, 7, 1279–1288. [Google Scholar] [CrossRef]
- Jiao, C.; Tan, X.; Lin, A.; Yang, W. Preparation of Activated Carbon Supported Bead String Structure Nano Zero Valent Iron in a Polyethylene Glycol-Aqueous Solution and Its Efficient Treatment of Cr(VI) Wastewater. Molecules 2020, 25, 47. [Google Scholar] [CrossRef] [Green Version]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Bioresour. Technol. 2013, 134, 94–100. [Google Scholar] [CrossRef]
- Bayazit, Ş.S.; Kerkez, Ö. Hexavalent chromium adsorption on superparamagnetic multi-wall carbon nanotubes and activated carbon composites. Chem. Eng. Res. Des. 2014, 92, 2725–2733. [Google Scholar] [CrossRef]
- Zhu, J.; Gu, H.; Guo, J.; Chen, M.; Wei, H.; Luo, Z.; Colorado, H.A.; Yerra, N.; Ding, D.; Ho, T.C.; et al. Mesoporous magnetic carbon nanocomposite fabrics for highly efficient Cr(vi) removal. J. Mater. Chem. A 2014, 2, 2256–2265. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Shi, J.; Zhao, M.; Yin, W.; Wang, X.; Wang, S.; Zhang, C. Carbon matrix of biochar from biomass modeling components facilitates electron transfer from zero-valent iron to Cr(VI). Environ. Sci. Pollut. Res. 2021, 29, 24309–24321. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.-J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. South Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Baikousi, M.; Daikopoulos, C.; Georgiou, Y.; Bourlinos, A.; Zbořil, R.; Deligiannakis, Y.; Karakassides, M.A. Novel Ordered Mesoporous Carbon with Innate Functionalities and Superior Heavy Metal Uptake. J. Phys. Chem. C 2013, 117, 16961–16971. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nada, A.; Kamal, D.E. Density functional theory and FTIR spectroscopic study of carboxyl group. Indian J. Pure Appl. Phys. 2005, 43, 911–917. [Google Scholar]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Sawalha, M.F.; Peralta-Videa, J.R.; Saupe, G.B.; Dokken, K.M.; Gardea-Torresdey, J.L. Using FTIR to corroborate the identity of functional groups involved in the binding of Cd and Cr to saltbush (Atriplex canescens) biomass. Chemosphere 2007, 66, 1424–1430. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Gardea-Torresdey, J.L.; Tiemann, K.J.; Armendariz, V.; Bess-Oberto, L.; Chianelli, R.R.; Rios, J.; Parsons, J.G.; Gamez, G. Characterization of Cr(VI) binding and reduction to Cr(III) by the agricultural byproducts of Avena monida (Oat) biomass. J. Hazard. Mater. 2000, 80, 175–188. [Google Scholar] [CrossRef]
- Kong, X.; Han, Z.; Zhang, W.; Song, L.; Li, H. Synthesis of zeolite-supported microscale zero-valent iron for the removal of Cr6+ and Cd2+ from aqueous solution. J. Environ. Manag. 2016, 169, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Chapman & Hall: London, UK, 1971. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]












| Material | SgBET (m2/g) | SCPSM (m2/g) | Vpore (cm3/g) | VCPSMmicro (% cm3/g) | VD-Rmicro (% cm3/g) |
|---|---|---|---|---|---|
| mAC-snack | 426 | 571 | 0.357 | 56 | 55 |
| mAC-snack (p.t.) | 544 | 754 | 0.303 | 76 | 86 |
| AC-snack | 766 | 1059 | 0.394 | 92 | 93 |
| Material | DCPSMNmean * (nm) | DCPSMVmean ** (nm) | DCPSMVmicro (nm) | DCPSMVmeso1 (nm) | DCPSMVmeso2 (nm) | DDFTVmicro (nm) | DDFTVmeso (nm) |
|---|---|---|---|---|---|---|---|
| mAC-snack | 1.52 | 25 | 1.44 | 10.7 | 66.8 | 0.71/1.2 | 5.3 |
| mAC-snack (p.t.) | 1.39 | 38 | 1.30 | 2.1 | 16.5 | 0.70/1.1 | 5.1 |
| AC-snack | 1.41 | 2 | 1.38 | 2.0 | 8.2 | 0.69/1.02 | 2.1 |
| Material | PFO R2 | PSO R2 | k2 gAC·mgCr(Vi)−1·min−1 | qe mgCr(VI)·gAC−1 | ri mgCr(VI)·gAC−1·min−1 |
|---|---|---|---|---|---|
| mAC-snack | 0.9568 | 0.9900 | 0.00221 | 13.77 | 0.4190 |
| mAC-snack (p.t.) | 0.9366 | 0.98673 | 6.2966 × 10−4 | 15.75 | 0.1562 |
| AC-snack | 0.9570 | 0.9895 | 5.0076 × 10−4 | 14.80 | 0.1097 |
| Material | R2 | qmax (mgCr(VI)/gAC) | KL (ppm−1) |
|---|---|---|---|
| Langmuir | 0.94944 | 88.382 | 0.27987 |
| Freundlich | 0.92982 | - | |
| Temkin | 0.89601 | - | |
| Redlich-Peterson | 0.93406 | - |
| Cinit. (ppm) | qe (Langm.) (mgCr(VI)/gAC) | PFO R2 | PSO R2 | k2 (g·mg−1·min−1) | ri2 (mg·g−1·min−1) |
|---|---|---|---|---|---|
| 2.41 | 10.62 | 0.95675 | 0.98995 | 0.00221 | 0.2493 |
| 5.24 | 22.38 | 0.87858 | 0.95114 | 5.25274 × 10−4 | 0.2631 |
| 10.88 | 42.23 | 0.82838 | 0.9527 | 1.75396 × 10−4 | 0.3128 |
| 19.81 | 62.43 | 0.73202 | 0.8664 | 8.94774 × 10−5 | 0.3487 |
| 39.65 | 77.58 | 0.4922 | 0.63204 | 9.57968 × 10−5 | 0.5766 |
| 49.31 | 80.17 | - | - | ||
| 68.72 | 82.88 | - | - |
| Adsorbent | pH | qm (mg/g) | Ref. |
|---|---|---|---|
| Sodium polyacrylate AC/Fe-Fe3C | 3 | 90 | [38] |
| Fe/Fe3C nanoparticles | 3 | 100 | [64] |
| nZVI–Fe3O4 | 3 | 100 | [65] |
| AC-fiber/nZVI | 3 | 91.5 | [66] |
| Filtrasorb 400-AC/nZVI | 4 | 25 | [67] |
| AC/nZVI | 5 | 24 | [68] |
| AC/Fe-Fe3O4 | 2–6 | 165–73 | [69] |
| modified AC/nZVI | 4 | 66 | [70] |
| Corn cob-AC/Fe3O4 | 2 | 57 | [71] |
| MWCNTs/Fe3O4 | 2 | 14.28 | [72] |
| AC/Fe3O4 | 2 | 2.84 | [72] |
| Magnetic nanocomposite prepared with cotton | 4 | 3.74 | [73] |
| Magnetic biochar prepared from Melia azedarach wood | 3 | 25.27 | [74] |
| Cellulose-biochar/ZVI | 3 | 30.83 | [75] |
| Hemicellulose-biochar/ZVI | 3 | 23.77 | [75] |
| Lignin-biochar/ZVI | 3 | 17.68 | [75] |
| Powdered AC | 4 | 46.9 | [76] |
| coconut tree sawdust-AC | 3 | 3.46 | [21] |
| longan seed-AC | 3 | 35.02 | [77] |
| Casuarina equisetifolia leaves-AC | 3 | 17.2 | [25] |
| Poseidonia Oceanica-AC | 3 | 120 | [24] |
| spent coffee-AC | 3 | 109 | [29] |
| mAC-snack | 3 | 88.38 | This work |
| Sample | T | Component (Color) | IS | Γ/2 | 2ε or QS | Bhf | ΔBhf | Area |
|---|---|---|---|---|---|---|---|---|
| (K) | (mm/s) | (mm/s) | (mm/s) | (kOe) | (kOe) | (%) | ||
| mAC-snack | 300 | α-Fe (red) | −0.01 | 0.14 | 0.01 | 330 | 3 | 49 |
| Fe3C (blue) | 0.20 | 0.14 | 0.04 | 206 | 8 | 45 | ||
| SPM Fe3+ oxide/hydroxide (green) | 0.20 | 0.25 | 0.74 | 0 | 0 | 6 | ||
| 77 | α-Fe (red) | 0.13 | 0.14 | 0.01 | 336 | 3 | 51 | |
| Fe3C (blue) | 0.33 | 0.14 | 0.01 | 254 | 13 | 46 | ||
| SPM Fe3+ oxide/hydroxide (green) | 0.33 | 0.25 | 0.80 | 0 | 0 | 3 | ||
| mAC-snack-Cr(VI) | 300 | α-Fe (red) | −0.01 | 0.14 | 0.02 | 331 | 2 | 24 |
| Fe3C (blue) | 0.19 | 0.14 | 0.04 | 206 | 9 | 30 | ||
| COL Fe3+ oxide/hydroxide (orange) | 0.34 | 0.15 | 0.00 | 260 | 184 | 29 | ||
| SPM Fe3+ oxide/hydroxide (green) | 0.39 | 0.29 | 0.79 | 0 | 0 | 17 | ||
| 77 | α-Fe (red) | 0.12 | 0.14 | 0.02 | 337 | 1 | 24 | |
| Fe3C (blue) | 0.33 | 0.14 | 0.00 | 245 | 7 | 30 | ||
| COL Fe3+ oxide/hydroxide (orange) | 0.42 | 0.15 | −0.03 | 296 | 136 | 31 | ||
| SPM Fe3+ oxide/hydroxide (green) | 0.48 | 0.23 | 0.73 | 0 | 0 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baikousi, M.; Moustaklis, K.; Karakassides, A.; Asimakopoulos, G.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Douvalis, A.P.; Salmas, C.E.; Karakassides, M.A. Use of a Hybrid Porous Carbon Material Derived from Expired Polysaccharides Snack/Iron Salt Exhibiting Magnetic Properties, for Hexavalent Chromium Removal. Polysaccharides 2022, 3, 326-346. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020019
Baikousi M, Moustaklis K, Karakassides A, Asimakopoulos G, Moschovas D, Avgeropoulos A, Bourlinos AB, Douvalis AP, Salmas CE, Karakassides MA. Use of a Hybrid Porous Carbon Material Derived from Expired Polysaccharides Snack/Iron Salt Exhibiting Magnetic Properties, for Hexavalent Chromium Removal. Polysaccharides. 2022; 3(2):326-346. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020019
Chicago/Turabian StyleBaikousi, Maria, Konstantinos Moustaklis, Angeliki Karakassides, Georgios Asimakopoulos, Dimitrios Moschovas, Apostolos Avgeropoulos, Athanasios B. Bourlinos, Alexios P. Douvalis, Constantinos E. Salmas, and Michael A. Karakassides. 2022. "Use of a Hybrid Porous Carbon Material Derived from Expired Polysaccharides Snack/Iron Salt Exhibiting Magnetic Properties, for Hexavalent Chromium Removal" Polysaccharides 3, no. 2: 326-346. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020019