Human Bone Marrow Mesenchymal Stromal Cell-Derived CXCL12, IL-6 and GDF-15 and Their Capacity to Support IgG-Secreting Cells in Culture Are Divergently Affected by Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Ex Vivo Culture of Human BM Derived MSCs
2.2. Cell Microscopy, Intracellular Doxo and Immunophenotyping by Flow Cytometry
2.3. Cytokine and Chemokine Array and Protein Network Analysis
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. In Vitro Differentiation of ASCs and Co-Culture with BM MSCs
2.6. ELISA and ELISPOT
2.7. Statistics
3. Results
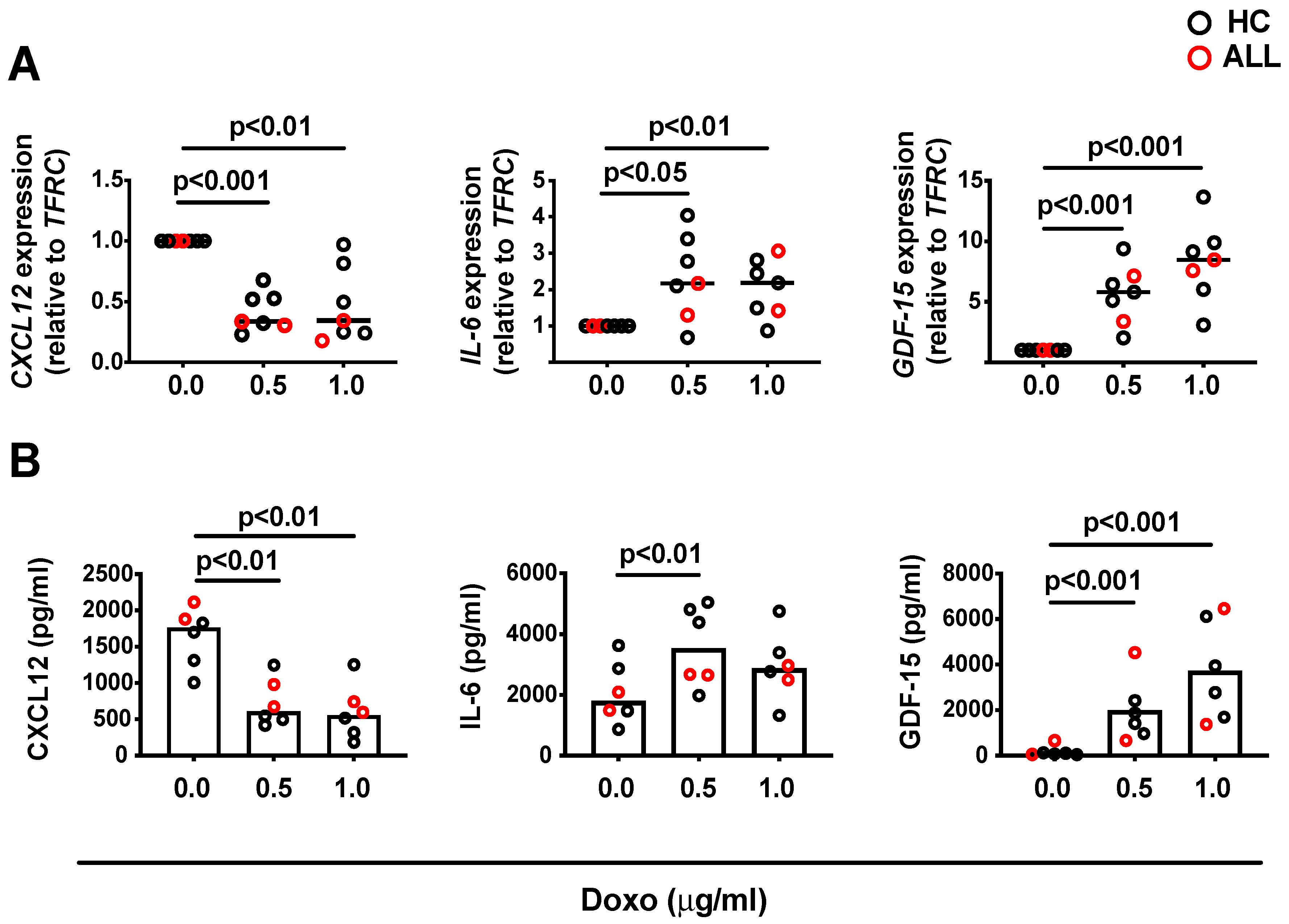
3.1. Characteristics of Human BM MSCs Following Exposure to Anthracycline
3.2. Doxo-Exposed Human BM MSCs Retain the Capacity to Support IgG-ASCs In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Owen, M.; Friedenstein, A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988, 136, 42–60. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S.; Schütz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Zacharaki, D.; Li, H.; Scheding, S. Human primary bone marrow stromal cells—basic biology and isolation strategies. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 26–34. [Google Scholar]
- Minges Wols, H.A.; Underhill, G.H.; Kansas, G.S.; Witte, P.L. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 2002, 169, 4213–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokoyoda, K.; Egawa, T.; Sugiyama, T.; Choi, B.-I.; Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 2004, 20, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Belnoue, E.; Tougne, C.; Rochat, A.-F.; Lambert, P.-H.; Pinschewer, D.D.; Siegrist, C.-A. Homing and adhesion patterns determine the cellular composition of the bone marrow plasma cell niche. J. Immunol. 2012, 188, 1283–1291. [Google Scholar] [CrossRef]
- Chu, V.T.; Berek, C. The establishment of the plasma cell survival niche in the bone marrow. Immunol. Rev. 2013, 251, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Anthony, B.A.; Link, D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodadadi, L.; Cheng, Q.; Radbruch, A.; Hiepe, F. The maintenance of memory plasma cells. Front. Immunol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdan, M.; Cren, M.; Robert, N.J.; Bollore, K.; Fest, T.; Duperray, C.; Guilloton, F.; Hose, D.; Tarte, K.; Klein, B.M. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 2014, 28, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, D.C.; Hyman, P.L.; Lu, T.T.; Ngo, V.N.; Bidgol, A.; Suzuki, G.; Zou, Y.-R.; Littman, D.R.; Cyster, J.G. A Coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 2001, 194, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, V.T.; Fröhlich, A.; Steinhauser, G.; Scheel, T.; Roch, T.T.; Fillatreau, S.S.; Lee, J.J.; Löhning, M.; Berek, C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011, 12, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Winter, O.; Moser, K.; Mohr, E.; Zotos, D.; Kaminski, H.; Szyska, M.; Roth, K.; Wong, D.M.; Dame, C.; Tarlinton, D.M.; et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 2010, 116, 1867–1875. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef]
- Kaushansky, K.; Zhan, H. The marrow stem cell niche in normal and malignant hematopoiesis. Ann. N. Y. Acad. Sci. 2020, 1466, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Rème, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef]
- Corre, J.; Hébraud, B.; Bourin, P. Concise review: Growth differentiation factor 15 in pathology: A clinical role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef]
- Banfi, A.; Bianchi, G.; Galotto, M.; Cancedda, R.; Quarto, R. Bone marrow stromal damage after chemo/radiotherapy: Occurrence, consequences and possibilities of treatment. Leuk. Lymphoma 2001, 42, 863–870. [Google Scholar] [CrossRef]
- Nilsson, A.; De Milito, A.; Engström, P.; Nordin, M.; Narita, M.; Grillner, L.; Chiodi, F.; Björk, O. Current chemotherapy protocols for childhood acute lymphoblastic leukemia induce loss of humoral immunity to viral vaccination antigens. Pediatrics 2002, 109, e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochennek, K.; Allwinn, R.; Langer, R.; Becker, M.; Keppler, O.T.; Klingebiel, T.; Lehrnbecher, T. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine 2014, 32, 3357–3361. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Chemaly, R.F. Practical review of immunizations in adult patients with cancer. Hum. Vaccines Immunother. 2015, 11, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghafian-Hedengren, S.; Söderström, I.; Sverremark-Ekström, E.; Nilsson, A. Insights into defective serological memory after acute lymphoblastic leukaemia treatment: The role of the plasma cell survival niche, memory B-cells and gut microbiota in vaccine responses. Blood Rev. 2018, 32, 71–80. [Google Scholar] [CrossRef]
- Thörnerup, I.; Forestier, E.; Botling, J.; Thuresson, B.; Wasslavik, C.; Björklund, E.; Li, A.; Lindström-Eriksson, E.; Malec, M.; Grönlund, E.; et al. Minimal residual disease assessment in childhood acute lymphoblastic leukaemia: A Swedish multi-centre study comparing real-time polymerase chain reaction and multicolour flow cytometry. Br. J. Haematol. 2011, 152, 743–753. [Google Scholar] [CrossRef]
- Sundin, M.; Ringdén, O.; Sundberg, B.; Nava, S.; Götherström, C.; Le Blanc, K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 2007, 92, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.; Caraux, A.; De Vos, J.; Fiol, G.; Larroque, M.; Cognot, C.; Bret, C.; Duperray, C.; Hose, D.; Klein, B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood 2009, 114, 5173–5181. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.C.; Garimalla, S.; Xiao, H.; Kyu, S.; Albizua, I.; Galipeau, J.; Chiang, K.-Y.; Waller, E.K.; Wu, R.; Gibson, G.; et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Addo, R.K.; Heinrich, F.; Heinz, G.A.; Schulz, D.; Sercan-Alp, Ö.; Lehmann, K.; Tran, C.L.; Bardua, M.; Matz, M.; Löhning, M.; et al. Single-cell transcriptomes of murine bone marrow stromal cells reveal niche-associated heterogeneity. Eur. J. Immunol. 2019, 49, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Pihlgren, M.; Schallert, N.; Tougne, C.; Bozzotti, P.; Kovarik, J.; Fulurija, A.; Kosco-Vilbois, M.; Lambert, P.-H.; Siegrist, C.-A. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur. J. Immunol. 2001, 31, 939–946. [Google Scholar] [CrossRef]
- Galotto, M.; Berisso, G.; Delfino, L.; Podesta, M.; Ottaggio, L.; Dallorso, S.; Dufour, C.; Ferrara, G.B.; Abbondandolo, A.; Dini, G.; et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp. Hematol. 1999, 27, 1460–1466. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Mueller, L.P.; Luetzkendorf, J.; Mueller, T.; Reichelt, K.; Simon, H.; Schmoll, H.-J. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: Evidence of resistance to apoptosis induction. Stem Cells 2006, 24, 2753–2765. [Google Scholar] [CrossRef]
- Li, J.; Law, H.K.W.; Lau, Y.L.; Chan, G.C.F. Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. Br. J. Haematol. 2004, 127, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, R.; Baumann, P.; Emmerich, B.; Meinhardt, G. Evaluation of chemosensitivity of human bone marrow stromal cells--differences between common chemotherapeutic drugs. Anticancer Res. 2006, 26, 347–350. [Google Scholar] [PubMed]
- Patel, K.J.; Trédan, O.; Tannock, I.F. Distribution of the anticancer drugs doxorubicin, mitoxantrone and topotecan in tumors and normal tissues. Cancer Chemother. Pharmacol. 2013, 72, 127–138. [Google Scholar] [CrossRef]
- Rusetskaya, N.V.; Khariton, N.; Yurchenko, O.V.; Chekhun, V.F. Distribution and accumulation of liposomal form of doxorubicin in breast cancer cells of MCF-7 line. Exp. Oncol. 2011, 33, 78–82. [Google Scholar] [PubMed]
- Yang, F.; Chen, H.; Liu, Y.; Yin, K.; Wang, Y.; Li, X.; Wang, G.; Wang, S.; Tan, X.; Xu, C.; et al. Doxorubicin caused apoptosis of mesenchymal stem cells via p38, JNK and p53 pathway. Cell. Physiol. Biochem. 2013, 32, 1072–1082. [Google Scholar] [CrossRef]
- Cruet-Hennequart, S.; Prendergast, Á.M.; Shaw, G.; Barry, F.P.; Carty, M.P. Doxorubicin induces the DNA damage response in cultured human mesenchymal stem cells. Int. J. Hematol. 2012, 96, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozhukharova, I.; Zemelko, V.; Kovaleva, Z.; Alekseenko, L.; Lyublinskaya, O.; Nikolsky, N. Therapeutic doses of doxorubicin induce premature senescence of human mesenchymal stem cells derived from menstrual blood, bone marrow and adipose tissue. Int. J. Hematol. 2018, 107, 286–296. [Google Scholar] [CrossRef]
- Grund, L.Z.; Komegae, E.N.; Lopes-Ferreira, M.; Lima, C. IL-5 and IL-17A are critical for the chronic IgE response and differentiation of long-lived antibody-secreting cells in inflamed tissues. Cytokine 2012, 59, 335–351. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Joyner, C.J.; Sanz, I.; Lee, F.E.-H. Factors affecting early antibody secreting cell maturation into long-lived plasma cells. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zehentmeier, S.; Roth, K.; Cseresnyes, Z.; Sercan, , Ö; Horn, K.; Niesner, R.A.; Chang, H.-D.; Radbruch, A.; Hauser, A.E. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur. J. Immunol. 2014, 44, 2306–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasaviciute, G.; Höbinger, A.; Ujvari, D.; Salamon, D.; Yusuf, A.; Sundin, M.; Sverremark-Ekström, E.; Chikhi, R.; Nilsson, A.; Saghafian-Hedengren, S. Human Bone Marrow Mesenchymal Stromal Cell-Derived CXCL12, IL-6 and GDF-15 and Their Capacity to Support IgG-Secreting Cells in Culture Are Divergently Affected by Doxorubicin. Hemato 2021, 2, 154-166. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010009
Lasaviciute G, Höbinger A, Ujvari D, Salamon D, Yusuf A, Sundin M, Sverremark-Ekström E, Chikhi R, Nilsson A, Saghafian-Hedengren S. Human Bone Marrow Mesenchymal Stromal Cell-Derived CXCL12, IL-6 and GDF-15 and Their Capacity to Support IgG-Secreting Cells in Culture Are Divergently Affected by Doxorubicin. Hemato. 2021; 2(1):154-166. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010009
Chicago/Turabian StyleLasaviciute, Gintare, Anna Höbinger, Dorina Ujvari, Daniel Salamon, Aisha Yusuf, Mikael Sundin, Eva Sverremark-Ekström, Rayan Chikhi, Anna Nilsson, and Shanie Saghafian-Hedengren. 2021. "Human Bone Marrow Mesenchymal Stromal Cell-Derived CXCL12, IL-6 and GDF-15 and Their Capacity to Support IgG-Secreting Cells in Culture Are Divergently Affected by Doxorubicin" Hemato 2, no. 1: 154-166. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010009






