A Carbon-Free Way for Obtaining Nanoscale Silicon
Abstract
:1. Introduction
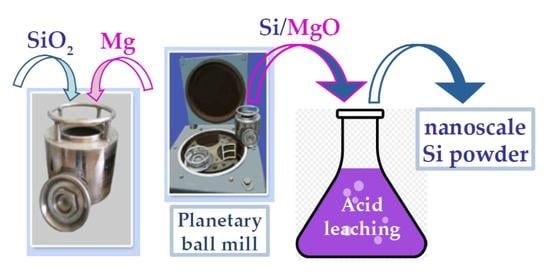
2. Materials and Methods
3. Results
3.1. Study of the MA SHS Reduction of Silica by Magnesium
- Molar ratio of Mg:SiO2 = 1.5:1;
- Molar ratio of Mg:SiO2 = 2:1, stoichiometry;
- Molar ratio of Mg:SiO2 = 2.5:1;
- Molar ratio of Mg:SiO2 = 3:1;
- Molar ratio of Mg:SiO2 = 4:1.
3.2. Study of the Mechanochemical Reduction of Silica by Magnesium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the kinetics of crystalline silicon nanoparticle lithiation with in situ transmission electron microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef]
- Timmerman, D.; Izeddin, I.; Stallinga, P.; Yassievich, I.N.; Gregorkiewicz, T. Space separated quantum cutting with silicon nanocrystals for photovoltaic applications. Nat. Photon. 2008, 2, 105–109. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef]
- Dai, F.; Zai, J.; Yi, R.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, D. Bottom-up synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance. Nat. Commun. 2014, 5, 3605. [Google Scholar] [CrossRef]
- Mason, B.A.; Groven, L.J.; Son, S.F.; Yetter, R.A. Combustion performance of several nanosilicon-based nanoenergetics. J. Propul. Power 2013, 29, 1435–1444. [Google Scholar] [CrossRef]
- Bux, S.K.; Blair, R.G.; Gogna, P.K.; Lee, H.; Chen, G.; Dresselhaus, M.S.; Kaner, R.B.; Fleurial, J.P. Nanostructured bulk silicon as an effective thermoelectric material. Adv. Funct. Mater. 2009, 19, 2445–2452. [Google Scholar] [CrossRef]
- Stoetzel, J.; Schneider, T.; Mueller, M.M.; Kleebe, H.J.; Wiggers, H.; Schierning, G.; Schmechel, R. Microstructure and thermoelectric properties of Si-WSi2 nanocomposites. Acta Mater. 2017, 125, 321–326. [Google Scholar] [CrossRef]
- Gribov, B.G.; Zinov’ev, K.V. Preparation of high-purity silicon for solar cells. Inorg. Mater. 2003, 39, 653–662. [Google Scholar] [CrossRef]
- Merzhanov, A. Self-propagating high-temperature synthesis: Twenty years of search and findings. In Combustion and Plasma Synthesis of High-Temperature Materials; Munir, Z., Holt, J., Eds.; VCH: New York, NY, USA, 1990; pp. 1–53. [Google Scholar]
- Merzhanov, A. Combustion and Synthesis of Materials; ISMAN Publ.: Chernogolovka, Russia, 1998; 511p. [Google Scholar]
- Itin, V.; Naiborodenko, Y. High-Temperature Synthesis of Intermetallic Compounds; TSU Publ.: Tomsk, Russia, 1989; 218p. [Google Scholar]
- Zakaryan, M.K.; Aydinyan, S.V.; Kharatyan, S.L. Preparation of Fine-grained Silicon from Serpentine Mineral by Magnesiothermic Reduction of Silica in the Presence of Reaction Products as Diluents. Silicon 2017, 9, 841–846. [Google Scholar] [CrossRef]
- Merzhanov, A.; Mukasyan, A. Combustion of Solid Flame; Tonus Press: Moscow, Russia, 2007; 336p. [Google Scholar]
- Moore, J.J.; Feng, H.J. Combustion Synthesis of Advanced Materials: Part I. Reaction Parameters. Prog. Mater. Sci. 1995, 39, 243–273. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principles and applications. Adv. Chem. Eng. 1998, 24, 79–226. [Google Scholar]
- Rogachev, A.S.; Mukasyan, A.S. Combustion for Material Synthesis; CRC Press: Boca Raton, FL, USA, 2014; 424p. [Google Scholar] [CrossRef]
- Talako, T. Powders Obtained by the Method of Mechanically Activated Self-Propagating High-Temperature Synthesis for Heat-Resistant, Wear-Resistant and Radio-Absorbing Gas-Thermal Coatings. Ph.D. Thesis, State Scientific and Production Association of Powder Metallurgy, Minsk, Belarus, 2015. [Google Scholar]
- Haouli, S.; Boudebane, S.; Slipper, I.J.; Lemboub, S.; Gębara, P.; Mezrag, S. Combustion synthesis of silicon by magnesiothermic reduction. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 280–287. [Google Scholar] [CrossRef]
- Zulumyan, N.H.; Isahakyan, A.R.; Hovhannisyan, Z.H.; Torosyan, A.R. The influence of mechanical activation on the process of thermal reduction of silica by magnesium powder. In Magnesium Technology; Luo, A., Neelameggham, N., Beals, R., Eds.; The Minerals, Metals & Materials Society (TMS): Pittsburgh, PA, USA, 2006; pp. 351–354. [Google Scholar]
- Grigorieva, T.; Korchagin, M.; Lyakhov, N. Combination of SHS and mechanochemical synthesis for nanopowder technologies. KONA Powder Part. J. 2002, 20, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Korchagin, M. Experimental Investigation of the Mechanism of Interaction of Reagents of Self-Propagating High-Temperature Synthesis and Development of Scientific Foundations for the Production of Nanocomposite Materials with a Ceramic-Hardened Phase. Ph.D. Thesis, Altai Polzunov State University, Barnaul, Russia, 2007. [Google Scholar]
- Lyakhov, N.; Talako, T.; Grigoreva, T. Influence of Mechanical Activation on Processes of Phase and Structure Formation by Self-Propagating High-Temperature Synthesis; Parallel: Novosibirsk, Russia, 2008; 168p. [Google Scholar]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; 413p. [Google Scholar]
- McCormick, P.G. Application of mechanical alloying to chemical refining. Mater. Trans. JIM 1995, 36, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, G.B.; McCormick, P.G. Displacement reactions during mechanical alloying. Metall. Trans. A 1990, 21, 2789–2794. [Google Scholar] [CrossRef]
- Matteazzi, P.; LeCaër, G. Synthesis of nanocrystalline alumina-metal composites by room-temperature ball-milling of metal oxides and aluminium. J. Am. Ceram. Soc. 1992, 75, 2749–2755. [Google Scholar] [CrossRef]
- Yang, H.; McCormick, P.G. Mechanochemical reduction of V2O5. J. Solid State Chem. 1994, 110, 136–141. [Google Scholar] [CrossRef]
- Mukopadhyay, D.K.; Prisbrey, K.A.; Suryanarayana, C.; Froyes, F.H. Ball-milling, a novel extraction process for production of W from WO3 using magnesium as reductant. In Tungsten and Refractory Metals 3; Bose, A., Dowding, R., Eds.; Metal Powder Industries Federation: New York, NY, USA, 1996; pp. 239–346. [Google Scholar]
- El-Eskandarany, M.S.; El-Bahnasawy, H.N.; Ahmed, H.A.; Eissa, N.A. Mechanical solid-state reduction of haematite with magnesium. J. Alloys Compd. 2001, 314, 286–295. [Google Scholar] [CrossRef]
- Grigorieva, T.F.; Sharafutdinov, M.R.; Kaminskii, Y.D.; Vorsina, I.A.; Barinova, A.P.; Lyakhov, N.Z.; Talako, T.L.; Tsibulya, S.V. Ultrafine Si/Al2O3 composites obtained by combining methods of mechanical activation and self-propagating high-temperature synthesis. Combust. Explos. Shock. Waves 2010, 46, 36–40. [Google Scholar] [CrossRef]
- Raschman, P.; Fedoro¢ková, A. Study of inhibiting effect of acid concentration on the dissolution rate of magnesium oxide during the leaching of dead-burned magnesite. Hydrometallurgy 2004, 71, 403–412. [Google Scholar] [CrossRef]
- Smith, A. Applied Infrared Spectroscopy; Wiley: New York, NY, USA, 1979; 332p. [Google Scholar]
- Gates-Rector, S.; Blanton, T. Powder Diffraction File PDF4+ ICDD Release; Cambridge University Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.P.G. Thermitic Thermodynamics; CRC Press: Boca Raton, FL, USA, 2020; 1094p. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigorieva, T.F.; Bokhonov, B.B.; Sharafutdinov, M.R.; Barinova, A.P.; Lyakhov, N.Z. Solid-State Combustion in Mechanically Activated SHS Systems. I. Effect of Activation Time on Process Parameters and Combustion Product Composition. Combust. Explos. Shock. Waves 2003, 39, 43–50. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigorieva, T.F.; Bokhonov, B.B.; Sharafutdinov, M.R.; Barinova, A.P.; Lyakhov, N.Z. Solid-State Combustion in Mechanically Activated SHS Systems. II. Effect of Mechanical Activation Conditions on Process Parameters and Combustion Product Composition. Combust. Explos. Shock. Waves 2003, 39, 51–58. [Google Scholar] [CrossRef]
- Takacs, L. Reduction of magnetite by aluminum: A displacement reaction induced by mechanical alloying. Mater. Lett. 1992, 13, 119–124. [Google Scholar] [CrossRef]
- Schaffer, G.B.; McCormic, P.G. Combustion synthesis by mechanical alloying. Scr. Met. 1989, 23, 835–838. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigorieva, T.F.; Barinova, A.P.; Lyakhov, N.Z. The effect of mechanical treatment on the rate and limits of combustion in SHS processes. Intern. J. SHS 2000, 9, 307–320. [Google Scholar]
- Benjamin, J.S. Mechanical alloying. Sci. Am. 1976, 234, 40–58. [Google Scholar] [CrossRef]
- Gilman, P.S.; Benjamin, J.S. Mechanical alloying. Ann. Rev. Mater. Sci. 1983, 13, 279–300. [Google Scholar] [CrossRef]
- Koch, C.C. Materials synthesis by mechanical alloying. Ann. Rev. Mater. Sci. 1989, 19, 121–143. [Google Scholar] [CrossRef]
- Eckert, J.; Holzer, J.C.; Krill, C.E.; Johnson, W.L. Structural and Thermodynamic Properties of Nanocrystalline fcc Metals Prepared by Mechanical Attrition. J. Mat. Res. 1992, 7, 1751–1761. [Google Scholar] [CrossRef]
- Cabouro, G.; Chevalier, S.; Gaffet, E.; Vrel, D.; Boudet, N.; Bernard, F. In situ synchrotron investigation of MoSi2 formation mechanisms during current-activated SHS sintering. Acta Mater. 2007, 55, 6051–6063. [Google Scholar] [CrossRef]
- Grigoreva, T.F.; Kiseleva, T.Y.; Petrova, S.A.; Talako, T.L.; Vosmerikov, S.V.; Udalova, T.A.; Devyatkina, E.T.; Novakova, A.A.; Lyakhov, N.Z. Mechanochemically Stimulated Reactions of the Reduction of Iron Oxide with Aluminum. Phys. Met. Metallogr. 2021, 122, 572–578. [Google Scholar] [CrossRef]
- Kiseleva, T.; Letsko, A.; Talako, T.; Kovaleva, S.; Grigoreva, T.; Novakova, A.; Lyakhov, N. Mossbauer spectroscopy study of Fe/ZrO2 nanocomposites formation by MA SHS technology. Hyperfine Interact. 2018, 239, 14. [Google Scholar] [CrossRef]
- Grigoreva, T.F.; Šepelák, V.; Vorsina, I.A.; Letsko, A.I.; Talako, T.L.; Ilyushchenko, A.F.; Lyakhov, N.Z. Cu/ZrO2 composites prepared by self-propagating high-temperature processing of the mechanically pre-activated system. Powder Metall. Prog. 2011, 11, 284–289. [Google Scholar]
- Lidin, R.; Molochko, V.; Andreeva, L. Khimicheskie Svoistva Neorganicheskikh Veshchestv, 4th ed.; Kolos: Moscow, Russia, 2003; 480p. [Google Scholar]
- Veryatin, U.; Mashirev, V.; Ryabtsev, N.; Tarasov, V. Termodinamicheskie Svoistva Neorganicheskikh Veshchestv; Atomizdat: Moscow, Russia, 1965; 461p. [Google Scholar]
- Spravochnik Khimika, 2nd ed.; Khimiya: Moscow-Leningrad, Russia, 1966; Volume 1, pp. 802–803.
- Kiselev, A.D. Processes for the Production of Silicon with a Low Content of Impurities Using Magnetothermic Reduction of Silicon Dioxide in Tight Drop Apparatuses. Ph.D. Thesis, National Research Tomsk Polytechnic University, Tomsk, Russia, 2014. [Google Scholar]
- Pavlov, E.A.; Udalova, T.A.; Grigoreva, T.F.; Vosmerikov, S.V.; Vorsina, I.A.; Devyatkina, E.T.; Lyakhov, N.Z. Preparing Ultradisperse Copper Powder via the Mechanochemical Reduction of Copper Oxides by Magnesium. Bull. Russ. Acad. Sci. Phys. 2018, 82, 574–577. [Google Scholar] [CrossRef]
- Udalova, T.A.; Vosmerikov, S.V.; Grigoreva, T.F.; Devyatkina, E.T.; Lyakhov, N.Z. Fine Tungsten from W/MgO Mechanocomposite Obtained by the Reduction of Tungsten (VI) Oxide by Magnesium. Chem. Sustain. Dev. 2019, 27, 304–309. [Google Scholar] [CrossRef]
- Udalova, T.A.; Grigoreva, T.F.; Devyatkina, E.T.; Vosmerikov, S.V.; Lyakhov, N.Z. Mechanochemical Reduction of GeO2 with Magnesium. Chem. Sustain. Dev. 2018, 26, 537–542. [Google Scholar] [CrossRef]
- Lyakhov, N.Z.; Udalova, T.A.; Grigoreva, T.F. Mechanochemical way of obtaining highly dispersed metal powders. In Proceedings of the Nano-2020, 7th All-Russian Conference on Nanomaterials (NANO-2020), Moscow, Russia, 18–22 May 2020; IMET RAS Publish: Moscow, Russia, 2020; pp. 10–11. [Google Scholar]
- Udalova, T.A.; Vosmerikov, S.V.; Grigoreva, T.F.; Devyatkina, E.T.; Lyakhov, N.Z. Mechanochemical synthesis of highly dispersed tungsten and molybdenum. In Proceedings of the Thermodynamics and Materials Science, Abstracts of the 13th Symposium with International Participation Thermodynamics and Materials Science, Novosibirsk, Russia, 26–30 October 2020; Gelfond, N.V., Ed.; NIIC SB RAS Publish: Novosibirsk, Russia, 2020; p. 54. [Google Scholar]











| Impurity | Molar Ratio = 2:1 | Molar Ratio = 2.5:1 |
|---|---|---|
| Al | 0.02 | 0.02 |
| Ca | 0.08 | 0.07 |
| Cd | 0.03 | 0.01 |
| Cr | 0.02 | 0.02 |
| Fe | 0.05 | 0.03 |
| K | 0.2 | 0.14 |
| Mg | 1.1 | 0.08 |
| Mn | less than 0.01 | less than 0.01 |
| Ni | 0.03 | 0.04 |
| Zn | less than 0.01 | less than 0.01 |
| Si | 98.45 | 99.57 |
| No. | The Reactions | T = 25 °C | T = 627 °C | ||
|---|---|---|---|---|---|
| ΔH, kJ/mol | ΔG, kJ/mol | ΔH, kJ/mol | ΔG, kJ/mol | ||
| 1 | 2Mg + SiO2 → Si + 2MgO | −292.8 | −279 | −292.6 | −259 |
| 2 | 2Mg + 3SiO2 → 2MgSiO3 + Si | −367.3 | −370 | −372.2 | −346 |
| 3 | 4Mg + SiO2 → Mg2Si + 2MgO | −372.2 | −355 | −372.2 | −330 |
| 4 | 2Mg + Si → Mg2Si | −79.5 | −77 | −80.2 | −71 |
| 5 | MgO + SiO2 → MgSiO3 | −37.3 | −45 | −39.6 | −43 |
| 6 | 2MgO + SiO2 → Mg2SiO4 | −63 | −72 | −63.6 | −69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyakhov, N.; Grigoreva, T.; Talako, T.; Udalova, T.; Vosmerikov, S.; Devyatkina, E. A Carbon-Free Way for Obtaining Nanoscale Silicon. Powders 2022, 1, 18-32. https://0-doi-org.brum.beds.ac.uk/10.3390/powders1010003
Lyakhov N, Grigoreva T, Talako T, Udalova T, Vosmerikov S, Devyatkina E. A Carbon-Free Way for Obtaining Nanoscale Silicon. Powders. 2022; 1(1):18-32. https://0-doi-org.brum.beds.ac.uk/10.3390/powders1010003
Chicago/Turabian StyleLyakhov, Nikolay, Tatiana Grigoreva, Tatiana Talako, Tatyana Udalova, Sergey Vosmerikov, and Evgeniya Devyatkina. 2022. "A Carbon-Free Way for Obtaining Nanoscale Silicon" Powders 1, no. 1: 18-32. https://0-doi-org.brum.beds.ac.uk/10.3390/powders1010003