The Fate of Transplanted Olfactory Progenitors Is Conditioned by the Cell Phenotypes of the Receiver Brain Tissue in Cocultures
Abstract
:1. Introduction
2. Results
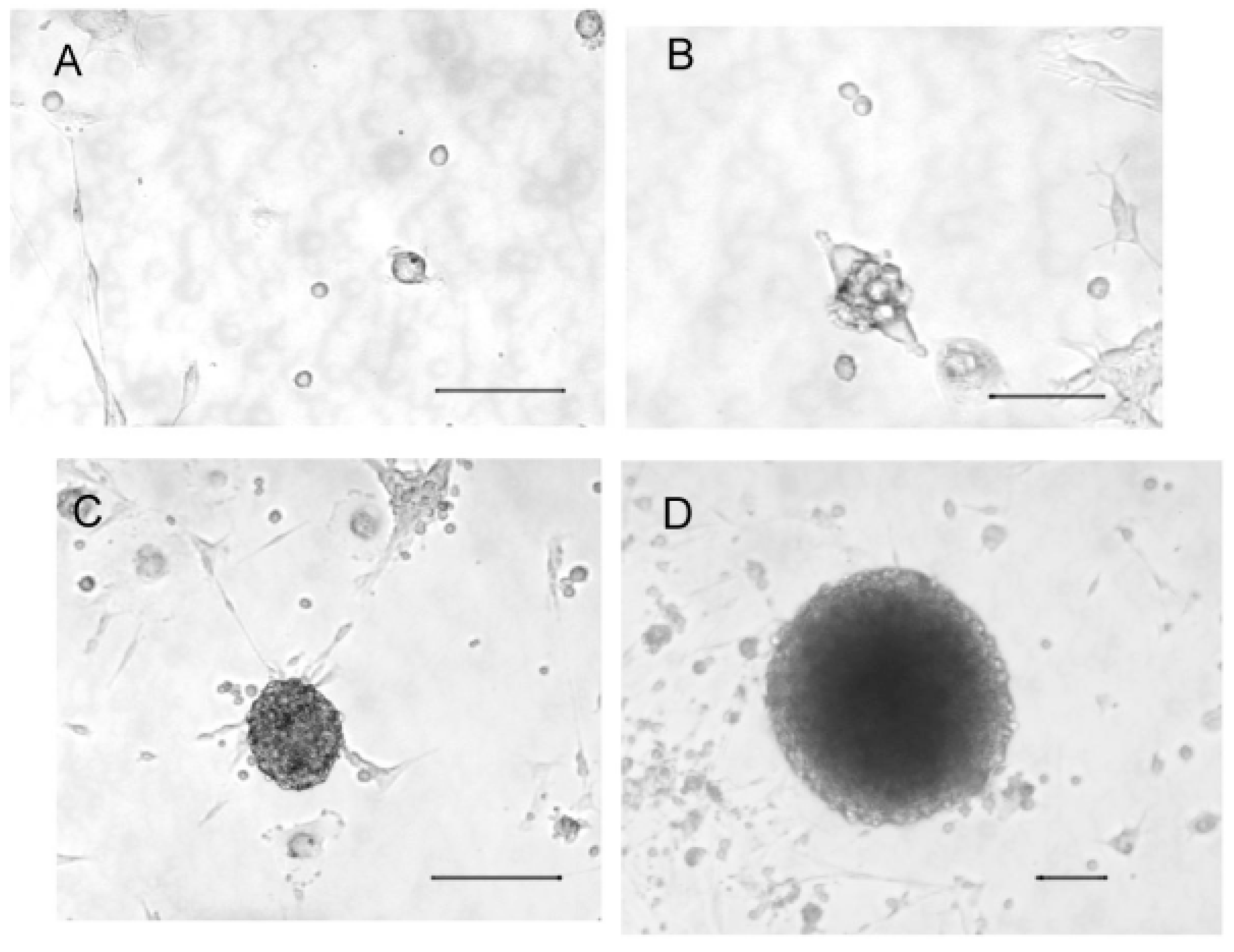
2.1. Cultured Olfactory Progenitors
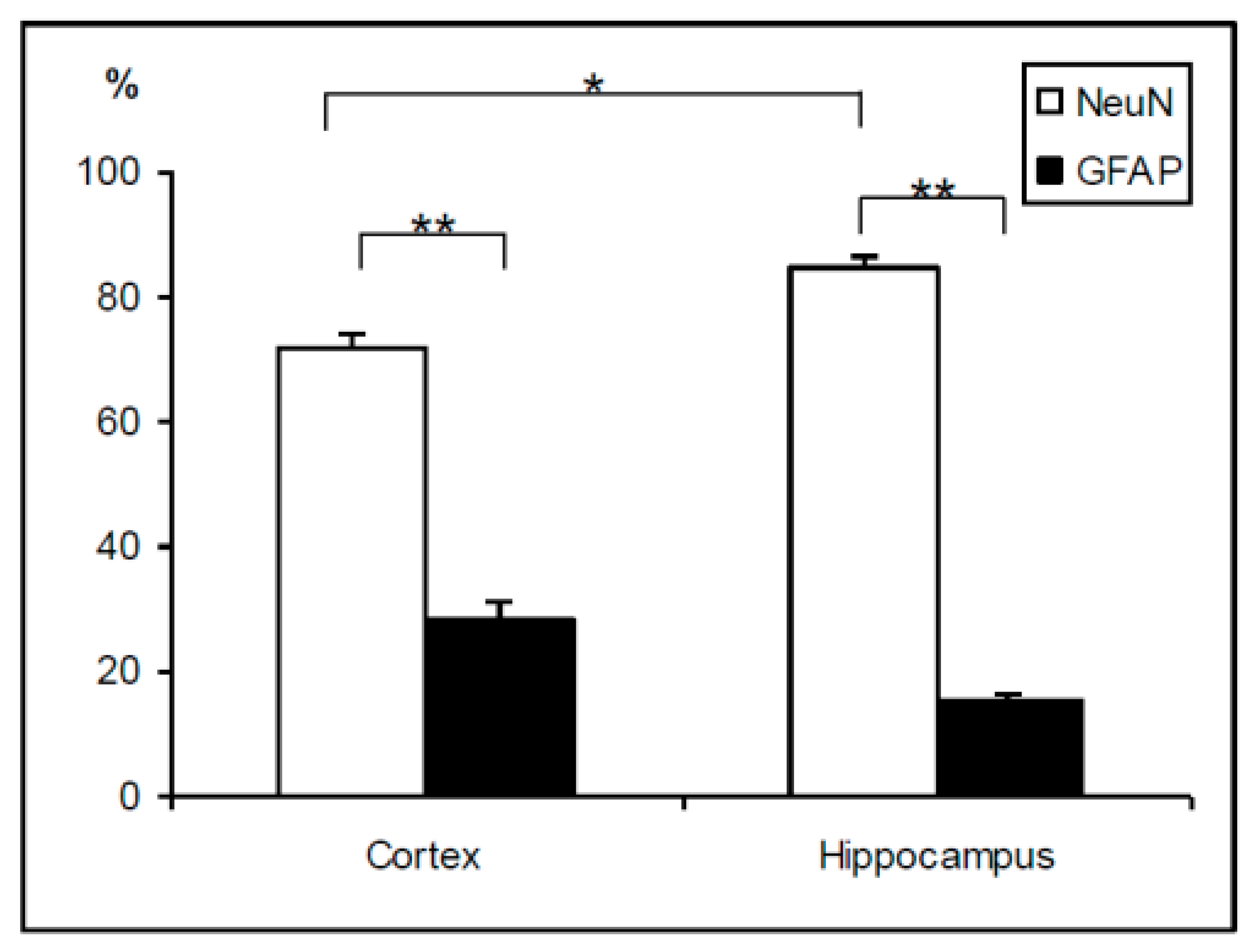
2.2. Cell Phenotypes in Olfactory Epithelium Cultures
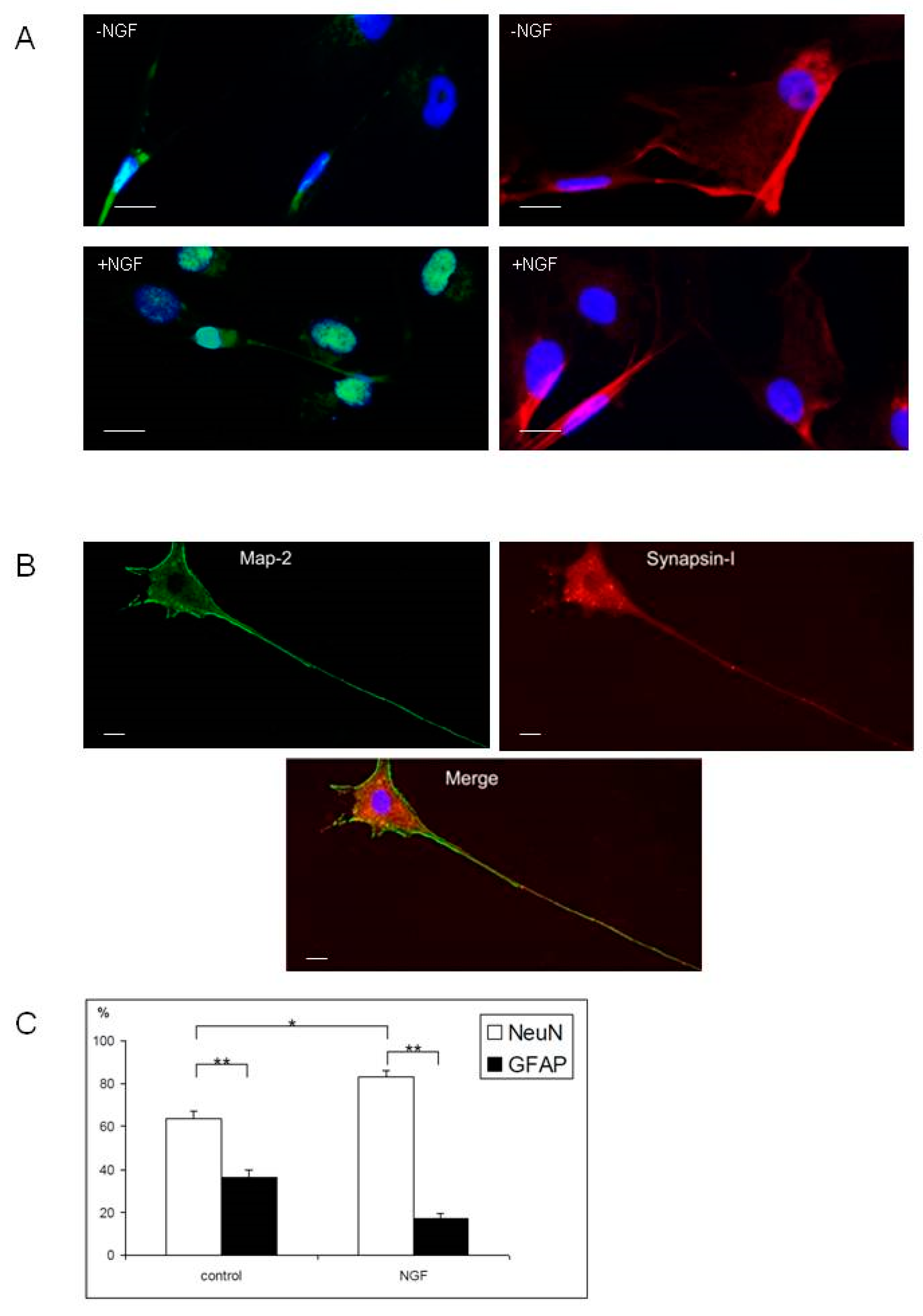
2.3. Differentiation of Olfactory Progenitors
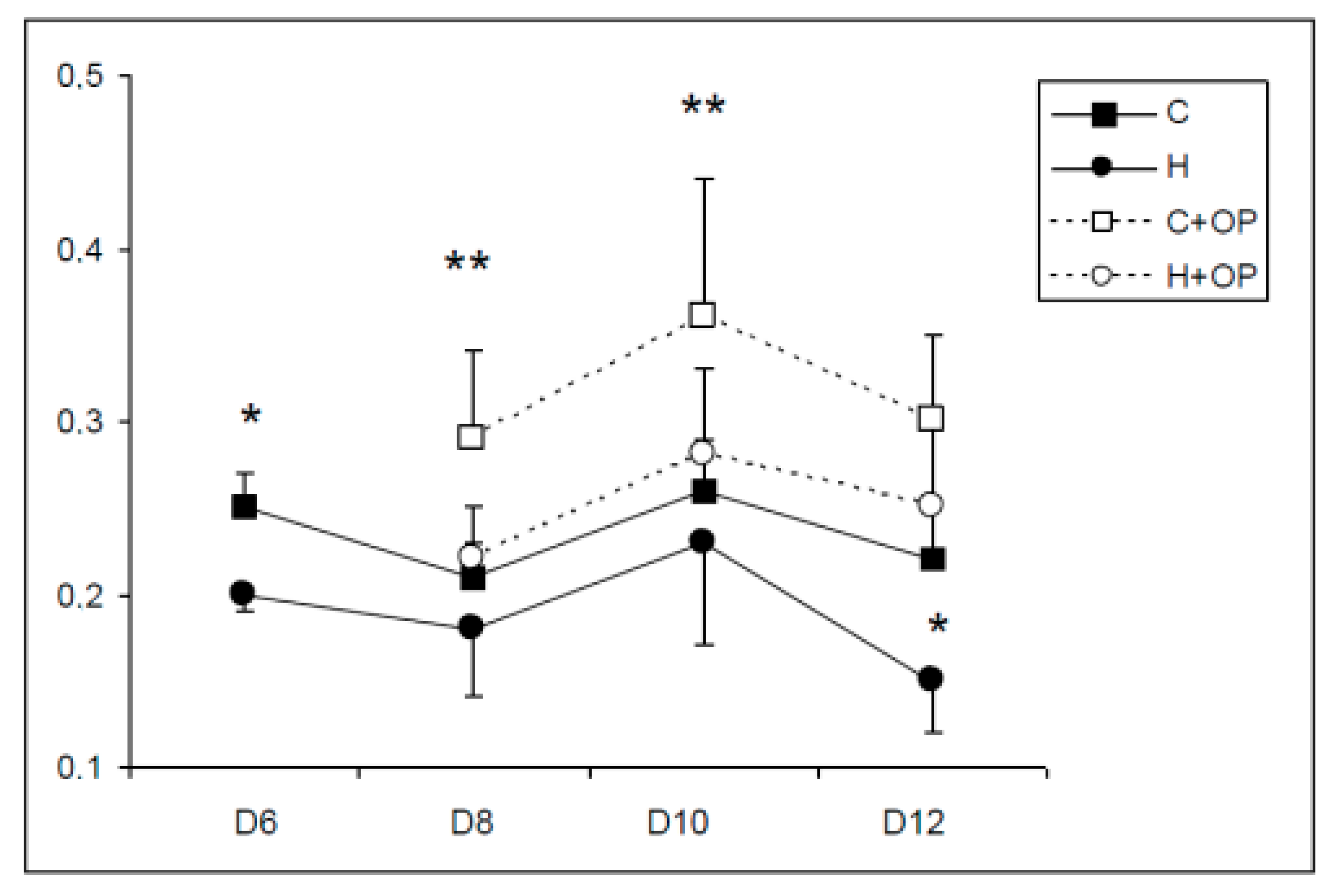
2.4. Viability of Primary Cultures and Cocultures
2.5. Olfactory Progenitor Differentiation in Cocultures
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Olfactory Progenitor Culture
4.3. Olfactory Progenitor Differentiation
4.4. Primary Cultures
4.5. Cocultures
4.6. Cell Viability
4.7. Cell Phenotypes
Author Contributions
Funding
Conflicts of Interest
References
- Heike, T.; Nakahata, T. Stem cell plasticity in the hematopoietic system. Int. J. Hematol. 2004, 79, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Brazelton, T.R.; Rossi, F.M.; Keshet, G.I.; Blau, H.M. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 2000, 290, 1775–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, C.; Bjorklund, A.; Wictorin, K. Neuronal differentiation following transplantation of expanded mouse neurosphere cultures derived from different embryonic forebrain regions. Exp. Neurol. 2003, 184, 615–635. [Google Scholar] [CrossRef]
- Lu, J.; Feron, F.; Mackay-Sim, A.; Waite, P.M. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 2002, 125, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, R.; Kurokawa, M.S.; Chiba, S.; Yoshikawa, H.; Ide, M.; Tadokoro, M.; Nito, S.; Nakatsuji, N.; Kondoh, Y.; Nagata, K.; et al. Transplantation of neural cells derived from retinoic acid-treated cynomolgus monkey embryonic stem cells successfully improved motor function of hemiplegic mice with experimental brain injury. Neurobiol. Dis. 2005, 20, 38–48. [Google Scholar] [CrossRef]
- Gao, J.; Prough, D.S.; McAdoo, D.J.; Grady, J.J.; Parsley, M.O.; Ma, L.; Tarensenko, Y.I.; Wu, P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 2006, 201, 281–292. [Google Scholar] [CrossRef]
- Bakshi, A.; Shimizu, S.; Keck, C.A.; Cho, S.; LeBold, D.G.; Morales, D.; Arenas, E.; Snyder, E.Y.; Watson, D.J.; McIntosh, T.K. Neural progenitor cells engineered to secrete GDNF show enhanced survival, neuronal differentiation and improve cognitive function following traumatic brain injury. Eur. J. Neurosci. 2006, 23, 2119–2134. [Google Scholar] [CrossRef]
- Xiao, M.; Klueber, K.M.; Guo, Z.; Lu, C.; Wang, H.; Roisen, F.J. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol. Dis. 2007, 26, 363–374. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Dulin, J.N.; Adler, A.F.; Kumamaru, H.; Poplawski, G.H.D.; Lee-Kubli, C.; Strobl, H.; Gibbs, D.; Kadoya, K.; Fawcett, J.W.; Lu, P.; et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun. 2018, 9, 84. [Google Scholar] [CrossRef]
- Christophersen, N.S.; Meijer, X.; Jorgensen, J.R.; Englund, U.; Gronborg, M.; Seiger, A.; Brundin, P.; Wahlberg, L.U. Induction of dopaminergic neurons from growth factor expanded neural stem/progenitor cell cultures derived from human first trimester forebrain. Brain Res. Bull. 2006, 70, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Matsumoto, Y.; Wang, Q.; Kawai, N.; Tamiya, T.; Nagao, S. Differences in the neuronal stem cells survival, neuronal differentiation and neurological improvement after transplantation of neural stem cells between mild and severe experimental traumatic brain injury. J. Med. Investig. 2006, 53, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, S.S.; Theofilopoulos, S.; Jauniaux, E.; Stern, G.M.; Bradford, H.F. The differentiation potential of human foetal neuronal progenitor cells in vitro. Brain Res. Dev. Brain Res. 2004, 153, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Timmer, M.; Grosskreutz, J.; Schlesinger, F.; Krampfl, K.; Wesemann, M.; Just, L.; Bufler, J.; Grothe, C. Dopaminergic properties and function after grafting of attached neural precursor cultures. Neurobiol. Dis. 2006, 21, 587–606. [Google Scholar] [CrossRef]
- Buzanska, L.; Jurga, M.; Domanska-Janik, K. Neuronal differentiation of human umbilical cord blood neural stem-like cell line. Neurodegener. Dis. 2006, 3, 19–26. [Google Scholar] [CrossRef]
- Habich, A.; Jurga, M.; Markiewicz, I.; Lukomska, B.; Bany-Laszewicz, U.; Domanska-Janik, K. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp. Hematol. 2006, 34, 914–925. [Google Scholar] [CrossRef]
- Mezey, E.; Chandross, K.J.; Harta, G.; Maki, R.A.; McKercher, S.R. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290, 1779–1782. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Gonzalez, X.R.; Keene, C.D.; Verfaillie, C.M.; Low, W.C. Neural induction of adult bone marrow and umbilical cord stem cells. Curr. Neurovasc. Res. 2004, 1, 207–213. [Google Scholar] [CrossRef]
- Bossolasco, P.; Cova, L.; Calzarossa, C.; Rimoldi, S.G.; Borsotti, C.; Deliliers, G.L.; Silani, V.; Soligo, D.; Polli, E. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp. Neurol. 2005, 193, 312–325. [Google Scholar] [CrossRef]
- Towns, C.R.; Jones, D.G. Stem cells, embryos, and the environment: A context for both science and ethics. J. Med. Ethics. 2004, 30, 410–413. [Google Scholar] [CrossRef] [Green Version]
- Daar, A.S.; Bhatt, A.; Court, E.; Singer, P.A. Stem cell research and transplantation: Science leading ethics. Transpl. Proc. 2004, 36, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Chan, S. Current and emerging global themes in the bioethics of regenerative medicine: The tangled web of stem cell translation. Regen. Med. 2017, 12, 839–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, C.; Bodo, E.; Petschnik, A.E.; Danner, S.; Tiede, S.; Paus, R. Towards the development of a pragmatic technique for isolating and differentiating nestin-positive cells from human scalp skin into neuronal and glial cell populations: Generating neurons from human skin? Exp. Dermatol. 2006, 15, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Feron, F.; Mackay-Sim, A.; Andrieu, J.L.; Matthaei, K.I.; Holley, A.; Sicard, G. Stress induces neurogenesis in non-neuronal cell cultures of adult olfactory epithelium. Neuroscience 1999, 88, 571–583. [Google Scholar] [CrossRef]
- Newman, M.P.; Feron, F.; Mackay-Sim, A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience 2000, 99, 343–350. [Google Scholar] [CrossRef]
- McCurdy, R.D.; Feron, F.; McGrath, J.J.; Mackay-Sim, A. Regulation of adult olfactory neurogenesis by insulin-like growth factor-I. Eur. J. Neurosci. 2005, 22, 1581–1588. [Google Scholar] [CrossRef]
- Othman, M.; Lu, C.; Klueber, K.; Winstead, W.; Roisen, F. Clonal analysis of adult human olfactory neurosphere forming cells. Biotech. Histochem. 2005, 80, 189–200. [Google Scholar] [CrossRef]
- Calof, A.L.; Bonnin, A.; Crocker, C.; Kawauchi, S.; Murray, R.C.; Shou, J.; Wu, H.H. Progenitor cells of the olfactory receptor neuron lineage. Microsc. Res. Tech. 2002, 58, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Winstead, W.; Marshall, C.T.; Lu, C.L.; Klueber, K.M.; Roisen, F.J. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am. J. Rhinol. 2005, 19, 83–90. [Google Scholar] [CrossRef]
- Zhang, X.; Klueber, K.M.; Guo, Z.; Lu, C.; Roisen, F.J. Adult human olfactory neural progenitors cultured in defined medium. Exp. Neurol. 2004, 186, 112–123. [Google Scholar] [CrossRef]
- Marshall, C.T.; Lu, C.; Winstead, W.; Zhang, X.; Xiao, M.; Harding, G.; Klueber, K.M.; Roisen, F.J. The therapeutic potential of human olfactory-derived stem cells. Histol. Histopathol. 2006, 21, 633–643. [Google Scholar] [PubMed]
- Krolewski, R.C.; Jang, W.; Schwob, J.E. The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo. Exp. Neurol. 2011, 229, 308–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Klueber, K.M.; Lu, C.; Guo, Z.; Marshall, C.T.; Wang, H.; Roisen, F.J. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp. Neurol. 2005, 194, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, J.; Klueber, K.M.; Guo, Z.; Lu, C.; Qiu, M.; Roisen, F.J. Induction of oligodendrocytes from adult human olfactory epithelial-derived progenitors by transcription factors. Stem Cells 2005, 23, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, J.; Klueber, K.M.; Guo, Z.; Lu, C.; Winstead, W.I.; Qiu, M.; Roisen, F.J. Role of transcription factors in motoneuron differentiation of adult human olfactory neuroepithelial-derived progenitors. Stem Cells 2006, 24, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Klueber, K.M.; Guo, Z.; Cai, J.; Lu, C.; Winstead, W.I.; Qiu, M.; Roisen, F.J. Induction of neuronal differentiation of adult human olfactory neuroepithelial-derived progenitors. Brain Res. 2006, 1073, 109–119. [Google Scholar] [CrossRef]
- Murrell, W.; Feron, F.; Wetzig, A.; Cameron, N.; Splatt, K.; Bellette, B.; Bianco, J.; Perry, C.; Lee, G.; Mackay-Sim, A. Multipotent stem cells from adult olfactory mucosa. Dev. Dyn. 2005, 233, 496–515. [Google Scholar] [CrossRef]
- Lima, C.; Pratas-Vital, J.; Escada, P.; Hasse-Ferreira, A.; Capucho, C.; Peduzzi, J.D. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J. Spinal Cord Med. 2006, 29, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Vaca, A.L.; Benitez-King, G.; Ruiz, V.; Ramírez-Rodríguez, G.B.; Hernández-de la Cruz, B.; Salamanca-Gómez, F.A.; González-Márquez, H.; Ramírez-Sánchez, I.; Ortíz-López, L.; Vélez-Del Valle, C.; et al. Exfoliated Human Olfactory Neuroepithelium: A Source of Neural Progenitor Cells. Mol. Neurobiol. 2018, 55, 2516–2523. [Google Scholar] [CrossRef]
- Hasan, U.; Singh, S.K. The Astrocyte-Neuron Interface: An Overview on Molecular and Cellular Dynamics Controlling Formation and Maintenance of the Tripartite Synapse. Methods Mol. Biol. 2019, 1938, 3–18. [Google Scholar]
- Kallur, T.; Darsalia, V.; Lindvall, O.; Kokaia, Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J. Neurosci. Res. 2006, 84, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Nat, R.; Nilbratt, M.; Narkilahti, S.; Winblad, B.; Hovatta, O.; Nordberg, A. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia 2007, 55, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Iwatsuki, K.; Shinzawa, K.; Ishihara, M.; Moriwaki, T.; Umegaki, M.; Kishima, H.; Yoshimine, T. Adult olfactory sphere cells are a source of oligodendrocyte and Schwann cell progenitors. Stem Cell Res. 2013, 11, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Iwatsuki, K.; Ishihara, M.; Shikina, T.; Shinzawa, K.; Moriwaki, T.; Ninomiya, K.; Ohkawa, T.; Umegaki, M.; Kishima, H.; et al. Isolation of human adult olfactory sphere cells as a cell source of neural progenitors. Stem Cell Res. 2015, 15, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Carter, L.A.; MacDonald, J.L.; Roskams, A.J. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J. Neurosci. 2004, 24, 5670–5683. [Google Scholar] [CrossRef]
- Marei, H.E.; Farag, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Lashen, S.; Rezk, S.; Pallini, R.; Casalbore, P.; Cenciarelli, C. Human olfactory bulb neural stem cells expressing hNGF restore cognitive deficit in Alzheimer’s disease rat model. J. Cell Physiol. 2015, 230, 116–130. [Google Scholar] [CrossRef]
- Ma, W.; Fitzgerald, W.; Liu, Q.Y.; O’Shaughnessy, T.J.; Maric, D.; Lin, H.J.; Alkon, D.L.; Barker, J.L. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp. Neurol. 2004, 190, 276–288. [Google Scholar] [CrossRef]
- Durbec, P.; Rougon, G. Transplantation of mammalian olfactory progenitors into chick hosts reveals migration and differentiation potentials dependent on cell commitment. Mol. Cell Neurosci. 2001, 17, 561–576. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Perera-Murcia, G.R.; Ortiz-López, L.; Vega-Rivera, N.M.; Babu, H.; García-Anaya, M.; González-Olvera, J.J. Vascular endothelial growth factor influences migration and focal adhesions, but not proliferation or viability, of human neural stem/progenitor cells derived from olfactory epithelium. Neurochem. Int. 2017, 108, 417–425. [Google Scholar] [CrossRef]
- Ortiz-López, L.; González-Olvera, J.J.; Vega-Rivera, N.M.; García-Anaya, M.; Carapia-Hernández, A.K.; Velázquez-Escobar, J.C.; Ramírez-Rodríguez, G.B. Human neural stem/progenitor cells derived from the olfactory epithelium express the TrkB receptor and migrate in response to BDNF. Neuroscience 2017, 355, 84–100. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Song, H.; Aimone, J.B.; Smrt, R.D.; Kuwabara, T.; Nakashima, K.; Gage, F.H.; Zhao, X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006, 15, 407–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, M.; Shiba, T.; Hasebe, S.; Ogita, K. Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J. Pharmacol. Sci. 2011, 115, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.J.; Oh, S.M.; Kwon, O.C.; Wulansari, N.; Lee, H.S.; Chang, M.Y.; Lee, E.; Sun, W.; Lee, S.E.; Chang, S.; et al. Cografting astrocytes improves cell therapeutic outcomes in a Parkinson’s disease model. J. Clin. Investig. 2018, 128, 463–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, R.Y. Creating a graft-friendly environment for stem cells in diseased brains. J. Clin. Investig. 2018, 128, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Tyers, P.; Borg, M.T.; Svendsen, C.N.; Dunnett, S.B.; Rosser, A.E. EGF and FGF-2 responsiveness of rat and mouse neural precursors derived from the embryonic CNS. Brain Res. Bull. 2005, 68, 83–94. [Google Scholar] [CrossRef]
- Feron, F.; Perry, C.; Cochrane, J.; Licina, P.; Nowitzke, A.; Urquhart, S.; Geraghty, T.; Mackay-Sim, A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 2005, 128, 2951–2960. [Google Scholar] [CrossRef] [Green Version]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem. Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef] [Green Version]
- Coutinho-Budd, J.C.; Sheehan, A.E.; Freeman, M.R. The secreted neurotrophin Spätzle 3 promotes glial morphogenesis and supports neuronal survival and function. Genes. Dev. 2017, 31, 2023–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Kale, S.; El Fatimy, R.; Rabinovsky, R.; Krichevsky, A.M. Co-cultures of Glioma Stem Cells and Primary Neurons, Astrocytes, Microglia, and Endothelial Cells for Investigation of Intercellular Communication in the Brain. Front. Neurosci. 2019, 13, 361. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibodies | Supplier | Working Dilution | Phenotype |
|---|---|---|---|
| Nestin | Chemicon | 1/300 | Undifferentiated cells |
| GFAP | Sigma | 1/200 | Astrocytes |
| NeuN | Chemicon | 1/300 | Mature neurons |
| Map-2 | Chemicon | 1/400 | Cytoplasmic and axonal microtubules in neurons |
| Synapsin-I | Chemicon | 1/400 | Cytoplasmic and axonal synaptic proteins in neurons |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grégory, P.; Nassila, A.; Jean-Louis, M.; Jean-Louis, G.; Jean-Luc, D.; Carine, B.-P. The Fate of Transplanted Olfactory Progenitors Is Conditioned by the Cell Phenotypes of the Receiver Brain Tissue in Cocultures. Int. J. Mol. Sci. 2020, 21, 7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197249
Grégory P, Nassila A, Jean-Louis M, Jean-Louis G, Jean-Luc D, Carine B-P. The Fate of Transplanted Olfactory Progenitors Is Conditioned by the Cell Phenotypes of the Receiver Brain Tissue in Cocultures. International Journal of Molecular Sciences. 2020; 21(19):7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197249
Chicago/Turabian StyleGrégory, Pourié, Akchiche Nassila, Millot Jean-Louis, Guéant Jean-Louis, Daval Jean-Luc, and Bossenmeyer-Pourié Carine. 2020. "The Fate of Transplanted Olfactory Progenitors Is Conditioned by the Cell Phenotypes of the Receiver Brain Tissue in Cocultures" International Journal of Molecular Sciences 21, no. 19: 7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197249





