The Cervicovaginal Mucus Barrier
Abstract
:1. Introduction
2. Cervicovaginal Mucus
2.1. Composition
2.1.1. Gel-Forming Mucins
2.1.2. Other Components
2.2. Hormonal Regulation
2.3. Functions
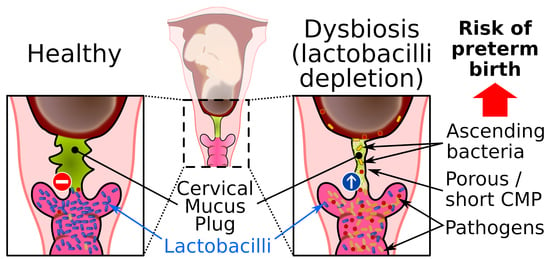
3. The Cervical Mucus Plug
4. The Vaginal Microbiota
4.1. Vaginal Lactobacilli
4.2. LAB Interactions within CVM
5. Bacterial Vaginal Infection
5.1. Vaginal Dysbiosis and Bacterial Vaginosis
5.2. Mucosal Barrier during Bacterial Vaginosis
5.3. Bacterial Vaginal Infection during Pregnancy
6. Mouse Models for the Study of CVM and the CMP
6.1. Anatomy and Physiology of the MGT
6.2. Vaginal Microbiota
6.3. Mouse Cervicovaginal Mucus
6.4. Transgenic Mouse Models to Study CVM
6.5. Mouse Models of Bacterial Vaginosis
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptide |
| CMP | cervical mucus plug |
| CVM | cervicovaginal mucus |
| CFU | colony-forming unit |
| CST | community state type |
| GFM | gel-forming mucin |
| HIV | human immunodeficiency virus |
| IL | interleukin |
| Ig | immunoglobulin |
| LAB | lactic acid bacteria |
| MGT | mouse genital tract |
| PAS | Periodic acid–Schiff |
| TFF | trefoil factor family |
| TNF | tumor necrosis factor |
| WGT | woman’s genital tract |
References
- Demouveaux, B.; Gouyer, V.; Gottrand, F.; Narita, T.; Desseyn, J.-L. Gel-forming mucin interactome drives mucus viscoelasticity. Adv. Colloid Interface Sci. 2018, 252, 69–82. [Google Scholar] [CrossRef]
- Elstein, M. Cervical mucus: Its physiological role and clinical significance. Adv. Exp. Med. Biol. 1982, 144, 301–318. [Google Scholar] [CrossRef]
- Elstein, M. Functions and physical properties of mucus in the female genital tract. Br. Med. Bull. 1978, 34, 83–88. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [Green Version]
- van de Wijgert, J.H.H.M.; Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017, 168, 589–864. [Google Scholar] [CrossRef]
- Becher, N.; Waldorf, K.A.; Hein, M.; Uldbjerg, N. The cervical mucus plug: Structured review of the literature. Acta Obstet. Gynecol. Scand. 2009, 88, 502–513. [Google Scholar] [CrossRef]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008, 70, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Desseyn, J.L.; Aubert, J.P.; Porchet, N.; Laine, A. Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol. 2000, 17, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Gipson, I.K.; Ho, S.B.; Spurr-Michaud, S.; Tisdale, A.S.; Zhan, Q.; Torlakovic, E.; Pudney, J.; Anderson, D.J.; Toribara, N.W.; Hill III, J.A. Mucin genes expressed by human female reproductive tract epithelia. Biol. Reprod. 1997, 56, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Alameda, F.; Mejías-Luque, R.; Garrido, M.; de Bolós, C. Mucin genes (MUC2, MUC4, MUC5AC, and MUC6) detection in normal and pathological endometrial tissues. Int. J. Gynecol. Pathol. 2007, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Habte, H.H.; de Beer, C.; Lotz, Z.E.; Tyler, M.G.; Schoeman, L.; Kahn, D.; Mall, A.S. The inhibition of the Human Immunodeficiency Virus type 1 activity by crude and purified human pregnancy plug mucus and mucins in an inhibition assay. Virol. J. 2008, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Desseyn, J.-L. Mucin CYS domains are ancient and highly conserved modules that evolved in concert. Mol. Phylogenet. Evol. 2009, 52, 284–292. [Google Scholar] [CrossRef]
- Gouyer, V.; Demouveaux, B.; Lacroix, G.; Valque, H.; Gottrand, F.; Desseyn, J.-L. Non-C-mannosylable mucin CYS domains hindered proper folding and secretion of mucin. Biochem. Biophys. Res. Commun. 2018, 506, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Perez-Vilar, J.; Randell, S.H.; Boucher, R.C. C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology 2004, 14, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Desseyn, J.-L.; Gouyer, V.; Gottrand, F. Biological modeling of mucus to modulate mucus barriers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G225–G227. [Google Scholar] [CrossRef] [Green Version]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Kaltner, H.; Abad-Rodríguez, J.; Corfield, A.P.; Kopitz, J.; Gabius, H.-J. The sugar code: Letters and vocabulary, writers, editors and readers and biosignificance of functional glycan-lectin pairing. Biochem. J. 2019, 476, 2623–2655. [Google Scholar] [CrossRef]
- Corfield, A.P.; Carroll, D.; Myerscough, N.; Probert, C.S. Mucins in the gastrointestinal tract in health and disease. Front. Biosci. 2001, 6, D1321–D1357. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P. The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human. Microorganisms 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Samson, M.H.; Chaiyarit, P.; Nortvig, H.; Vestergaard, E.M.; Ernst, E.; Nexo, E. Trefoil factor family peptides in human saliva and cyclical cervical mucus. Method evaluation and results on healthy individuals. Clin. Chem. Lab. Med. 2011, 49, 861–868. [Google Scholar] [CrossRef]
- Wiede, A.; Hinz, M.; Canzler, E.; Franke, K.; Quednow, C.; Hoffmann, W. Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res. 2001, 303, 109–115. [Google Scholar] [CrossRef]
- Hauser, F.; Poulsom, R.; Chinery, R.; Rogers, L.A.; Hanby, A.M.; Wright, N.A.; Hoffmann, W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 6961–6965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, K.M.; Malykhina, O.; Stieh, D.J.; Hope, T.J. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS ONE 2013, 8, e76176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; McKinley, S.A.; Wang, S.; Shi, F.; Mucha, P.J.; Forest, M.G.; Lai, S.K. Transient antibody-mucin interactions produce a dynamic molecular shield against viral invasion. Biophys. J. 2014, 106, 2028–2036. [Google Scholar] [CrossRef] [Green Version]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef]
- Brunelli, R.; Papi, M.; Arcovito, G.; Bompiani, A.; Castagnola, M.; Parasassi, T.; Sampaolese, B.; Vincenzoni, F.; De Spirito, M. Globular structure of human ovulatory cervical mucus. FASEB J. 2007, 21, 3872–3876. [Google Scholar] [CrossRef] [Green Version]
- Andersch-Björkman, Y.; Thomsson, K.A.; Holmén Larsson, J.M.; Ekerhovd, E.; Hansson, G.C. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell. Proteomics 2007, 6, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Ceric, F.; Silva, D.; Vigil, P. Ultrastructure of the human periovulatory cervical mucus. J. Electron. Microsc. 2005, 54, 479–484. [Google Scholar] [CrossRef]
- Grande, G.; Milardi, D.; Vincenzoni, F.; Pompa, G.; Biscione, A.; Astorri, A.L.; Fruscella, E.; De Luca, A.; Messana, I.; Castagnola, M.; et al. Proteomic characterization of the qualitative and quantitative differences in cervical mucus composition during the menstrual cycle. Mol. Biosyst. 2015, 11, 1717–1725. [Google Scholar] [CrossRef]
- Katz, D.F.; Slade, D.A.; Nakajima, S.T. Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Adv. Contracept. Off. J. Soc. Adv. Contracept. 1997, 13, 143–151. [Google Scholar] [CrossRef]
- Godley, M.J. Quantitation of vaginal discharge in healthy volunteers. Br. J. Obstet. Gynaecol. 1985, 92, 739–742. [Google Scholar] [CrossRef]
- Wolf, D.P.; Sokoloski, J.E.; Litt, M. Composition and functioin of human cervical mucus. Biochim. Biophys. Acta 1980, 630, 545–558. [Google Scholar] [CrossRef]
- Gilks, C.B.; Reid, P.E.; Clement, P.B.; Owen, D.A. Histochemical changes in cervical mucus-secreting epithelium during the normal menstrual cycle. Fertil. Steril. 1989, 51, 286–291. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Matsuura, F.; Moghissi, K.S. Structural studies of sialylated oligosaccharides of human midcycle cervical mucin. J. Biol. Chem. 1987, 262, 4733–4739. [Google Scholar]
- Gipson, I.K. Mucins of the human endocervix. Front. Biosci. 2001, 6, D1245–D1255. [Google Scholar] [CrossRef]
- Gipson, I.K.; Spurr-Michaud, S.; Moccia, R.; Zhan, Q.; Toribara, N.W.; Ho, S.B.; Gargiulo, A.R.; Hill, J.A. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol. Reprod. 1999, 60, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gipson, I.K.; Moccia, R.; Spurr-Michaud, S.; Argueso, P.; Gargiulo, A.R.; Hill, J.A., III; Offner, G.D.; Keutmann, H.T. The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J. Clin. Endocrinol. Metab. 2001, 86, 594–600. [Google Scholar] [CrossRef]
- Katz, D.F. Human cervical mucus: Research update. Am. J. Obstet. Gynecol. 1991, 165, 1984–1986. [Google Scholar] [CrossRef]
- Chrétien, F.C. Involvement of the glycoproteic meshwork of cervical mucus in the mechanism of sperm orientation. Acta Obstet. Gynecol. Scand. 2003, 82, 449–461. [Google Scholar]
- Lai, S.K.; Wang, Y.-Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Lai, S.K.; Ensign, L.M.; Zhong, W.; Cone, R.; Hanes, J. The microstructure and bulk rheology of human cervicovaginal mucus are remarkably resistant to changes in pH. Biomacromolecules 2013, 14, 4429–4435. [Google Scholar] [CrossRef] [Green Version]
- Witten, J.; Ribbeck, K. The particle in the spider’s web: Transport through biological hydrogels. Nanoscale 2017, 9, 8080–8095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witten, J.; Samad, T.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Radomsky, M.L.; Whaley, K.J.; Cone, R.A. Antibody diffusion in human cervical mucus. Biophys. J. 1994, 66, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Profet, M. Menstruation as a defense against pathogens transported by sperm. Q. Rev. Biol. 1993, 68, 335–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhurst, M.R.; Saltzman, W.M. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cell. Immunol. 1994, 156, 77–94. [Google Scholar] [CrossRef]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Critchfield, A.S.; Yao, G.; Jaishankar, A.; Friedlander, R.S.; Lieleg, O.; Doyle, P.S.; McKinley, G.; House, M.; Ribbeck, K. Cervical mucus properties stratify risk for preterm birth. PLoS ONE 2013, 8, e69528. [Google Scholar] [CrossRef] [Green Version]
- Smith-Dupont, K.B.; Wagner, C.E.; Witten, J.; Conroy, K.; Rudoltz, H.; Pagidas, K.; Snegovskikh, V.; House, M.; Ribbeck, K. Probing the potential of mucus permeability to signify preterm birth risk. Sci. Rep. 2017, 7, 10302. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Becher, N.; Bastholm, S.K.; Glavind, J.; Ramsing, M.; Kim, C.J.; Romero, R.; Jensen, J.S.; Uldbjerg, N. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet. Gynecol. Scand. 2014, 93, 102–108. [Google Scholar] [CrossRef]
- Loux, S.C.; Scoggin, K.E.; Troedsson, M.H.T.; Squires, E.L.; Ball, B.A. Characterization of the cervical mucus plug in mares. Reprod. Camb. Engl. 2017, 153, 197–210. [Google Scholar] [CrossRef]
- Palombi, C.; Paolucci, M.; Stradaioli, G.; Corubolo, M.; Pascolo, P.B.; Monaci, M. Evaluation of remote monitoring of parturition in dairy cattle as a new tool for calving management. BMC Vet. Res. 2013, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Owiny, J.R.; Fitzpatrick, R.J.; Spiller, D.G.; Dobson, H. Mechanical properties of the ovine cervix during pregnancy, labour and immediately after parturition. Br. Vet. J. 1991, 147, 432–436. [Google Scholar] [CrossRef]
- Hafez, E.S.; Jaszczak, S. Comparative anatomy and histology of the cervix uteri in non-human primates. Primates 1972, 13, 297–314. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Schmidt, B.; Failing, K.; Wehrend, A. Changes in the histomorphology of the canine cervix through the oestrous cycle. Theriogenology 2010, 74, 1075–1081.e1. [Google Scholar] [CrossRef]
- Malhi, J.S.; Gard, P.R.; Hanlon, G.W.; Marriott, C. The effects of bromhexine hydrochloride and S-carboxymethyl-L-cysteine on guinea-pig uterine microflora. J. Pharm. Pharmacol. 1987, 39, 1025–1028. [Google Scholar] [CrossRef]
- Pavlidis, I.; Spiller, O.B.; Sammut Demarco, G.; MacPherson, H.; Howie, S.E.M.; Norman, J.E.; Stock, S.J. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat. Commun. 2020, 11, 199. [Google Scholar] [CrossRef]
- Thompson, K.E.; Rayhon, S.L.; Bailey, G.; Delille, P.; McNerney, M.E. Assessment of cervical passage of vital dyes in pregnant, nonpregnant, and mated rats and mice. Reprod. Toxicol. 2016, 59, 1–7. [Google Scholar] [CrossRef]
- Bastholm, S.K.; Samson, M.H.; Becher, N.; Hansen, L.K.; Stubbe, P.R.; Chronakis, I.S.; Nexo, E.; Uldbjerg, N. Trefoil factor peptide 3 is positively correlated with the viscoelastic properties of the cervical mucus plug. Acta Obstet. Gynecol. Scand. 2017, 96, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef]
- Frew, L.; Makieva, S.; McKinlay, A.T.M.; McHugh, B.J.; Doust, A.; Norman, J.E.; Davidson, D.J.; Stock, S.J. Human cathelicidin production by the cervix. PLoS ONE 2014, 9, e103434. [Google Scholar] [CrossRef]
- Vornhagen, J.; Quach, P.; Santana-Ufret, V.; Alishetti, V.; Brokaw, A.; Armistead, B.; Qing Tang, H.; MacDonald, J.W.; Bammler, T.K.; Adams Waldorf, K.M.; et al. Human Cervical Mucus Plugs Exhibit Insufficiencies in Antimicrobial Activity Towards Group B Streptococcus. J. Infect. Dis. 2018, 217, 1626–1636. [Google Scholar] [CrossRef]
- Hein, M.; Petersen, A.C.; Helmig, R.B.; Uldbjerg, N.; Reinholdt, J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta Obstet. Gynecol. Scand. 2005, 84, 734–742. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [CrossRef] [Green Version]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Tsukumi, K.; Urano, T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp. Med. 2003, 53, 404–412. [Google Scholar]
- Vrbanac, A.; Riestra, A.M.; Coady, A.; Knight, R.; Nizet, V.; Patras, K.A. The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus. BMC Microbiol. 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGroarty, J.A. Cell surface appendages of lactobacilli. FEMS Microbiol. Lett. 1994, 124, 405–409. [Google Scholar] [CrossRef]
- Kovachev, S. Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 2018, 44, 31–39. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Crucitti, T. Eve’s garden: Myths, legends and secrets unmasked. Res. Microbiol. 2017, 168, 773–781. [Google Scholar] [CrossRef]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 6), 19–26. [Google Scholar]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [Green Version]
- Wilks, M.; Wiggins, R.; Whiley, A.; Hennessy, E.; Warwick, S.; Porter, H.; Corfield, A.; Millar, M. Identification and H(2)O(2) production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J. Clin. Microbiol. 2004, 42, 713–717. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Tachedjian, G.; O’Hanlon, D.E.; Ravel, J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Seta, F.; Parazzini, F.; De Leo, R.; Banco, R.; Maso, G.P.; De Santo, D.; Sartore, A.; Stabile, G.; Inglese, S.; Tonon, M.; et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 136–139. [Google Scholar] [CrossRef]
- Stapleton, A.E.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 1212–1217. [Google Scholar] [CrossRef]
- Vitali, B.; Abruzzo, A.; Parolin, C.; Palomino, R.A.Ñ.; Dalena, F.; Bigucci, F.; Cerchiara, T.; Luppi, B. Association of Lactobacillus crispatus with fructo-oligosaccharides and ascorbic acid in hydroxypropyl methylcellulose vaginal insert. Carbohydr. Polym. 2016, 136, 1161–1169. [Google Scholar] [CrossRef]
- Nunn, K.L.; Wang, Y.-Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15. [Google Scholar] [CrossRef] [Green Version]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Bansil, R.; Bhaskar, K.R.; Turner, B.S.; LaMont, J.T.; Niu, N.; Afdhal, N.H. pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys. J. 1999, 76, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.K.; Hida, K.; Shukair, S.; Wang, Y.-Y.; Figueiredo, A.; Cone, R.; Hope, T.J.; Hanes, J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009, 83, 11196–11200. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Hollinger, M.; Lachowicz-Scroggins, M.E.; Kerr, S.C.; Dunican, E.M.; Daniel, B.M.; Ghosh, S.; Erzurum, S.C.; Willard, B.; Hazen, S.L.; et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015, 7, 276ra27. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Sánchez, B.; Suárez, J.E.; Urdaci, M.C. Characterization of the adherence properties of human Lactobacilli strains to be used as vaginal probiotics. FEMS Microbiol. Lett. 2012, 328, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Leccese Terraf, M.C.; Mendoza, L.M.; Juárez Tomás, M.S.; Silva, C.; Nader-Macías, M.E.F. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J. Appl. Microbiol. 2014, 117, 1761–1772. [Google Scholar] [CrossRef]
- Zeng, Z.; Zuo, F.; Marcotte, H. Putative Adhesion Factors in Vaginal Lactobacillus gasseri DSM 14869: Functional Characterization. Appl. Environ. Microbiol. 2019, 85, e00800-19. [Google Scholar] [CrossRef] [Green Version]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus adhesion to mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D.; Lee, R.A.; Balen, A.H.; Rutherford, A.J. Bacterial vaginal flora in relation to changing oestrogen levels. Int. J. STD AIDS 2007, 18, 308–311. [Google Scholar] [CrossRef]
- Aslan, E.; Bechelaghem, N. To “douche” or not to “douche”: Hygiene habits may have detrimental effects on vaginal microbiota. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2018, 38, 678–681. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Thomas, K.K.; Agnew, K.; Ringwood, K. Prevalence and risks for bacterial vaginosis in women who have sex with women. Sex. Transm. Dis. 2010, 37, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Muzny, C.A.; Schwebke, J.R. Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses. J. Infect. Dis. 2016, 214 (Suppl. 1), S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Hooton, T.M. Recurrent urinary tract infection in women. Int. J. Antimicrob. Agents 2001, 17, 259–268. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Jespers, V.; Crucitti, T.; van de Wijgert, J.; Vaneechoutte, M.; Delany-Moretlwe, S.; Mwaura, M.; Agabe, S.; Menten, J. A DNA tool for early detection of vaginal dysbiosis in African women. Res. Microbiol. 2016, 167, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Brunham, R.C. Bacterial Vaginosis and Desquamative Inflammatory Vaginitis. N. Engl. J. Med. 2018, 379, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Hardy, L.; Cerca, N.; Jespers, V.; Vaneechoutte, M.; Crucitti, T. Bacterial biofilms in the vagina. Res. Microbiol. 2017, 168, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.; Toler, E.; DeLong, K.; Mafunda, N.A.; Bloom, S.M.; Zierden, H.C.; Moench, T.R.; Coleman, J.S.; Hanes, J.; Kwon, D.S.; et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020, 16, e1008236. [Google Scholar] [CrossRef] [Green Version]
- Eschenbach, D.A.; Davick, P.R.; Williams, B.L.; Klebanoff, S.J.; Young-Smith, K.; Critchlow, C.M.; Holmes, K.K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 1989, 27, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Olmsted, S.S.; Meyn, L.A.; Rohan, L.C.; Hillier, S.L. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Transm. Dis. 2003, 30, 257–261. [Google Scholar] [CrossRef]
- Moncla, B.J.; Chappell, C.A.; Debo, B.M.; Meyn, L.A. The Effects of Hormones and Vaginal Microflora on the Glycome of the Female Genital Tract: Cervical-Vaginal Fluid. PLoS ONE 2016, 11, e0158687. [Google Scholar] [CrossRef]
- Roberton, A.M.; Wiggins, R.; Horner, P.J.; Greenwood, R.; Crowley, T.; Fernandes, A.; Berry, M.; Corfield, A.P. A novel bacterial mucinase, glycosulfatase, is associated with bacterial vaginosis. J. Clin. Microbiol. 2005, 43, 5504–5508. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.G.; Robinson, L.S.; Gilbert, N.M.; Perry, J.C.; Lewis, A.L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 2013, 288, 12067–12079. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, F.; Wasinger, V.; Turroni, S.; Calanni, F.; Donders, G.; Brigidi, P.; Vitali, B. Proteome profiles of vaginal fluids from women affected by bacterial vaginosis and healthy controls: Outcomes of rifaximin treatment. J. Antimicrob. Chemother. 2013, 68, 2648–2659. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Alves, P.; Sousa, C.; Cereija, T.; França, Â.; Jefferson, K.K.; Cerca, N. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci. Rep. 2015, 5, 11640. [Google Scholar] [CrossRef] [Green Version]
- Cauci, S.; Monte, R.; Driussi, S.; Lanzafame, P.; Quadrifoglio, F. Impairment of the mucosal immune system: IgA and IgM cleavage detected in vaginal washings of a subgroup of patients with bacterial vaginosis. J. Infect. Dis. 1998, 178, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Campisciano, G.; Zanotta, N.; Licastro, D.; De Seta, F.; Comar, M. In vivo microbiome and associated immune markers: New insights into the pathogenesis of vaginal dysbiosis. Sci. Rep. 2018, 8, 2307. [Google Scholar] [CrossRef] [Green Version]
- Rebbapragada, A.; Howe, K.; Wachihi, C.; Pettengell, C.; Sunderji, S.; Huibner, S.; Ball, T.B.; Plummer, F.A.; Jaoko, W.; Kaul, R. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J. Acquir. Immune Defic. Syndr. 1999 2008, 49, 520–522. [Google Scholar] [CrossRef]
- Thurman, A.R.; Kimble, T.; Herold, B.; Mesquita, P.M.M.; Fichorova, R.N.; Dawood, H.Y.; Fashemi, T.; Chandra, N.; Rabe, L.; Cunningham, T.D.; et al. Bacterial Vaginosis and Subclinical Markers of Genital Tract Inflammation and Mucosal Immunity. AIDS Res. Hum. Retroviruses 2015, 31, 1139–1152. [Google Scholar] [CrossRef] [Green Version]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Cauci, S.; Driussi, S.; Monte, R.; Lanzafame, P.; Pitzus, E.; Quadrifoglio, F. Immunoglobulin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. Am. J. Obstet. Gynecol. 1998, 178, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Ciancimino, L.; Laganà, A.S.; Chiofalo, B.; Granese, R.; Grasso, R.; Triolo, O. Would it be too late? A retrospective case-control analysis to evaluate maternal-fetal outcomes in advanced maternal age. Arch. Gynecol. Obstet. 2014, 290, 1109–1114. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Beigi, R.H.; Yudin, M.H.; Cosentino, L.; Meyn, L.A.; Hillier, S.L. Cytokines, pregnancy, and bacterial vaginosis: Comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. J. Infect. Dis. 2007, 196, 1355–1360. [Google Scholar] [CrossRef]
- Cauci, S.; Culhane, J.F.; Di Santolo, M.; McCollum, K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am. J. Obstet. Gynecol. 2008, 198, 132.e1–132.e7. [Google Scholar] [CrossRef]
- Mattsby-Baltzer, I.; Platz-Christensen, J.J.; Hosseini, N.; Rosén, P. IL-1beta, IL-6, TNFalpha, fetal fibronectin, and endotoxin in the lower genital tract of pregnant women with bacterial vaginosis. Acta Obstet. Gynecol. Scand. 1998, 77, 701–706. [Google Scholar] [CrossRef]
- Platz-Christensen, J.J.; Brandberg, A.; Wiqvist, N. Increased prostaglandin concentrations in the cervical mucus of pregnant women with bacterial vaginosis. Prostaglandins 1992, 43, 133–134. [Google Scholar] [CrossRef]
- Platz-Christensen, J.J.; Mattsby-Baltzer, I.; Thomsen, P.; Wiqvist, N. Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am. J. Obstet. Gynecol. 1993, 169, 1161–1166. [Google Scholar] [CrossRef]
- Faught, B.M.; Reyes, S. Characterization and Treatment of Recurrent Bacterial Vaginosis. J. Womens Health 2019, 28, 1218–1226. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 1528. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Betsi, G.I.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007, 13, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Comparative Anatomy and Histology; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-381361-9.
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.G.; Onderdonk, A.B.; Drude, E.; Goldstein, C.; Anderka, M.; Alpert, S.; McCormack, W.M. Quantitative bacteriology of the vaginal flora. J. Infect. Dis. 1977, 136, 271–277. [Google Scholar] [CrossRef]
- Pascual, L.; Ruiz, F.; Giordano, W.; Barberis, I.L. Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. J. Med. Microbiol. 2010, 59, 360–364. [Google Scholar] [CrossRef]
- McGrory, T.; Garber, G.E. Mouse intravaginal infection with Trichomonas vaginalis and role of Lactobacillus acidophilus in sustaining infection. Infect. Immun. 1992, 60, 2375–2379. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, P.; Prabha, V. Evaluation of profertility effect of probiotic Lactobacillus plantarum 2621 in a murine model. Indian J. Med. Res. 2015, 142, 79–84. [Google Scholar] [CrossRef]
- Barfod, K.K.; Roggenbuck, M.; Hansen, L.H.; Schjørring, S.; Larsen, S.T.; Sørensen, S.J.; Krogfelt, K.A. The murine lung microbiome in relation to the intestinal and vaginal bacterial communities. BMC Microbiol. 2013, 13, 303. [Google Scholar] [CrossRef] [Green Version]
- Parr, E.L.; Bozzola, J.J.; Parr, M.B. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: Characterization of the immunoglobulins in vaginal mucus. J. Reprod. Immunol. 1998, 38, 15–30. [Google Scholar] [CrossRef]
- Portal, C.; Gouyer, V.; Magnien, M.; Plet, S.; Gottrand, F.; Desseyn, J.-L. In vivo imaging of the Muc5b gel-forming mucin. Sci. Rep. 2017, 7, e44591. [Google Scholar] [CrossRef] [Green Version]
- Desseyn, J.-L.; Portal, C.; Gottrand, F.; Gouyer, V. Transgenic Mouse Reporter to Study Muc5b In Vivo. Ann. Am. Thorac. Soc. 2018, 15, S149–S153. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L.H. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Amini, S.-E.; Gouyer, V.; Portal, C.; Gottrand, F.; Desseyn, J.-L. Muc5b is mainly expressed and sialylated in the nasal olfactory epithelium whereas Muc5ac is exclusively expressed and fucosylated in the nasal respiratory epithelium. Histochem. Cell Biol. 2019, 152, 167–174. [Google Scholar] [CrossRef]
- Valque, H.; Gouyer, V.; Duez, C.; Leboeuf, C.; Marquillies, P.; Le Bert, M.; Plet, S.; Ryffel, B.; Janin, A.; Gottrand, F.; et al. Muc5b-deficient mice develop early histological lung abnormalities. Biol. Open 2019, 8, bio046359. [Google Scholar] [CrossRef] [Green Version]
- Hasnain, S.Z.; Evans, C.; Roy, M.G.; Gallagher, A.L.; Kindrachuk, K.N.; Barron, L.; Dickey, B.F.; Wilson, M.S.; Wynn, T.A.; Grencis, R.K.; et al. Muc5ac: A critical component mediating the rejection of enteric nematodes. J. Exp. Med. 2011, 208, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Gouyer, V.; Dubuquoy, L.; Robbe-Masselot, C.; Neut, C.; Singer, E.; Plet, S.; Geboes, K.; Desreumaux, P.; Gottrand, F.; Desseyn, J.-L. Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier. Sci. Rep. 2015, 5, 9577. [Google Scholar] [CrossRef] [Green Version]
- Demouveaux, B.; Gouyer, V.; Robbe-Masselot, C.; Gottrand, F.; Narita, T.; Desseyn, J.-L. Mucin CYS domain stiffens the mucus gel hindering bacteria and spermatozoa. Sci. Rep. 2019, 9, 16993. [Google Scholar] [CrossRef] [PubMed]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Joncquel-Chevallier Curt, M.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C.; et al. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: The role of mucosal strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef]
- Ismail, M.N.; Stone, E.L.; Panico, M.; Lee, S.H.; Luu, Y.; Ramirez, K.; Ho, S.B.; Fukuda, M.; Marth, J.D.; Haslam, S.M.; et al. High-sensitivity O-glycomic analysis of mice deficient in core 2 {beta}1,6-N-acetylglucosaminyltransferases. Glycobiology 2011, 21, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.L.; Ismail, M.N.; Lee, S.H.; Luu, Y.; Ramirez, K.; Haslam, S.M.; Ho, S.B.; Dell, A.; Fukuda, M.; Marth, J.D. Glycosyltransferase function in core 2-type protein O glycosylation. Mol. Cell. Biol. 2009, 29, 3770–3782. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, J.E. Neisseria gonorrhoeae: Acquisition of auxotrophy in the mouse. Curr. Microbiol. 1978, 1, 253–256. [Google Scholar] [CrossRef]
- Amstey, M.S.; Kobos, K. An experimental model for disseminated herpesvirus infection of the neonate. Am. J. Obstet. Gynecol. 1976, 125, 40–44. [Google Scholar] [CrossRef]
- Young, J.A.; Cheung, K.S.; Lang, D.J. Infection and fertilization of mice after artificial insemination with a mixture of sperm and murine cytomegalovirus. J. Infect. Dis. 1977, 135, 837–840. [Google Scholar] [CrossRef]
- Hu, J.; Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Christensen, N.D. The Mouse Papillomavirus Infection Model. Viruses 2017, 9, 246. [Google Scholar] [CrossRef]
- Smith, J.S.; Herrero, R.; Bosetti, C.; Muñoz, N.; Bosch, F.X.; Eluf-Neto, J.; Castellsagué, X.; Meijer, C.J.L.M.; Van den Brule, A.J.C.; Franceschi, S.; et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 2002, 94, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Olmsted, S.S.; Khanna, K.V.; Ng, E.M.; Whitten, S.T.; Johnson, O.N.; Markham, R.B.; Cone, R.A.; Moench, T.R. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect. Dis. 2005, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Gu, Z.; Leneva, I.; Jiang, H.; Li, R.; Deng, L.; Yang, Z. The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis. Int. Immunopharmacol. 2019, 68, 58–67. [Google Scholar] [CrossRef]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat. Commun. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.B.; Araújo, P.F.; Bernal, F.M.; Rosa, A. de C.; Valente, S.A.; Teixeira, A.R.L. Sexual Transmission of American Trypanosomes from Males and Females to Naive Mates. J. Vis. Exp. JoVE 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-N.; Chuang, C.-M.; Chen, J.-Y.; Chieh-Yu, P. Epinecidin-1: A marine fish antimicrobial peptide with therapeutic potential against Trichomonas vaginalis infection in mice. Peptides 2019, 112, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tifrea, D.F.; de la Maza, L.M. Characterization of the Horizontal and Vertical Sexual Transmission of Chlamydia Genital Infections in a New Mouse Model. Infect. Immun. 2019, 87, e00834-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Gilbert, N.M.; Lewis, W.G.; Lewis, A.L. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS ONE 2013, 8, e59539. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, N.M.; Lewis, W.G.; Li, G.; Sojka, D.K.; Lubin, J.B.; Lewis, A.L. Gardnerella vaginalis and Prevotella bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1099–1108. [Google Scholar] [CrossRef]
- Racicot, K.; Cardenas, I.; Wünsche, V.; Aldo, P.; Guller, S.; Means, R.E.; Romero, R.; Mor, G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J. Immunol. 2013, 191, 934–941. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, R.; Martin-Fairey, C.; Sojka, D.K.; Herzog, E.D.; Jungheim, E.S.; Stout, M.J.; Fay, J.C.; Mahendroo, M.; Reese, J.; Herington, J.L.; et al. Mouse models of preterm birth: Suggested assessment and reporting guidelines. Biol. Reprod. 2018, 99, 922–937. [Google Scholar] [CrossRef] [Green Version]
- Akgul, Y.; Word, R.A.; Ensign, L.M.; Yamaguchi, Y.; Lydon, J.; Hanes, J.; Mahendroo, M. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J. Clin. Investig. 2014, 124, 5481–5489. [Google Scholar] [CrossRef]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Tangney, M.; Taylor, P.W.; Peebles, D.; Buckley, S.M.K.; et al. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am. J. Pathol. 2018, 188, 2164–2176. [Google Scholar] [CrossRef] [Green Version]
- Boozarjomehri, F.; Timor-Tritsch, I.; Chao, C.R.; Fox, H.E. Transvaginal ultrasonographic evaluation of the cervix before labor: Presence of cervical wedging is associated with shorter duration of induced labor. Am. J. Obstet. Gynecol. 1994, 171, 1081–1087. [Google Scholar] [CrossRef]
- Datta, J.; Palmer, M.J.; Tanton, C.; Gibson, L.J.; Jones, K.G.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.H.; et al. Prevalence of infertility and help seeking among 15,000 women and men. Hum. Reprod. Oxf. Engl. 2016, 31, 2108–2118. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, R.L. Chapter 22—Female Infertility. In Yen and Jaffe’s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 556–581.e7. ISBN 978-0-323-47912-7. [Google Scholar]
- Newby, J.M.; Seim, I.; Lysy, M.; Ling, Y.; Huckaby, J.; Lai, S.K.; Forest, M.G. Technological strategies to estimate and control diffusive passage times through the mucus barrier in mucosal drug delivery. Adv. Drug Deliv. Rev. 2018, 124, 64–81. [Google Scholar] [CrossRef] [Green Version]
- Ensign, L.M.; Tang, B.C.; Wang, Y.-Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4, 138ra79. [Google Scholar] [CrossRef] [Green Version]




| GFM | Tissue | |||||
|---|---|---|---|---|---|---|
| Name | CYS a | Bartholin’s | Cervix | Uterus | ||
| Glands | Ecto | Endo | CMP | |||
| MUC2 [9,10,11] | 2 | + | + | |||
| MUC5AC [9,10,11] | 9 | + | + | + | ||
| MUC5B [9,10,11] | 7 | + | + | |||
| MUC6 [9,10] | 0 | + | + | |||
| CST | WGT Dominated by | MGT Dominated by |
|---|---|---|
| CST I | L. crispatus | Staphylococcus |
| CST II | L. gasseri | Staphylococcus and Enterococcus |
| CST III | L. iners | Enterococcus and Lactobacillus |
| CST IV | Streptococcus | |
| CST IV-A | Strictly anaerobic bacteria | |
| CST IV-B | Atopobium and anaerobic | |
| bacteria | ||
| CST V | L. jensenii | Mix of bacteria |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.-L. The Cervicovaginal Mucus Barrier. Int. J. Mol. Sci. 2020, 21, 8266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218266
Lacroix G, Gouyer V, Gottrand F, Desseyn J-L. The Cervicovaginal Mucus Barrier. International Journal of Molecular Sciences. 2020; 21(21):8266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218266
Chicago/Turabian StyleLacroix, Guillaume, Valérie Gouyer, Frédéric Gottrand, and Jean-Luc Desseyn. 2020. "The Cervicovaginal Mucus Barrier" International Journal of Molecular Sciences 21, no. 21: 8266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218266