miR-27b Modulates Insulin Signaling in Hepatocytes by Regulating Insulin Receptor Expression
Abstract
:1. Introduction
2. Results
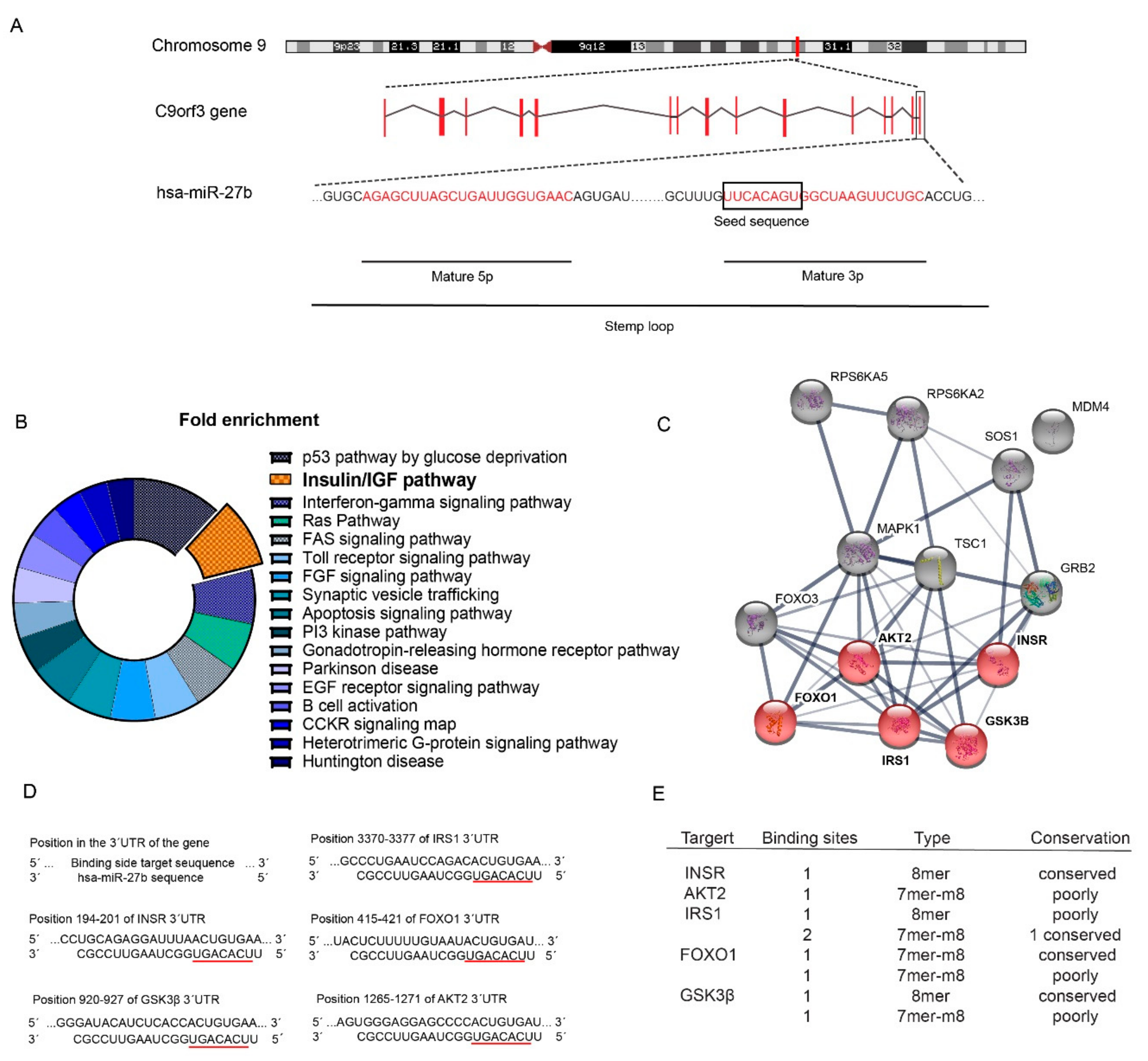
2.1. Identification of miR-27b as a Potential Regulator of the Insulin Signaling Pathway
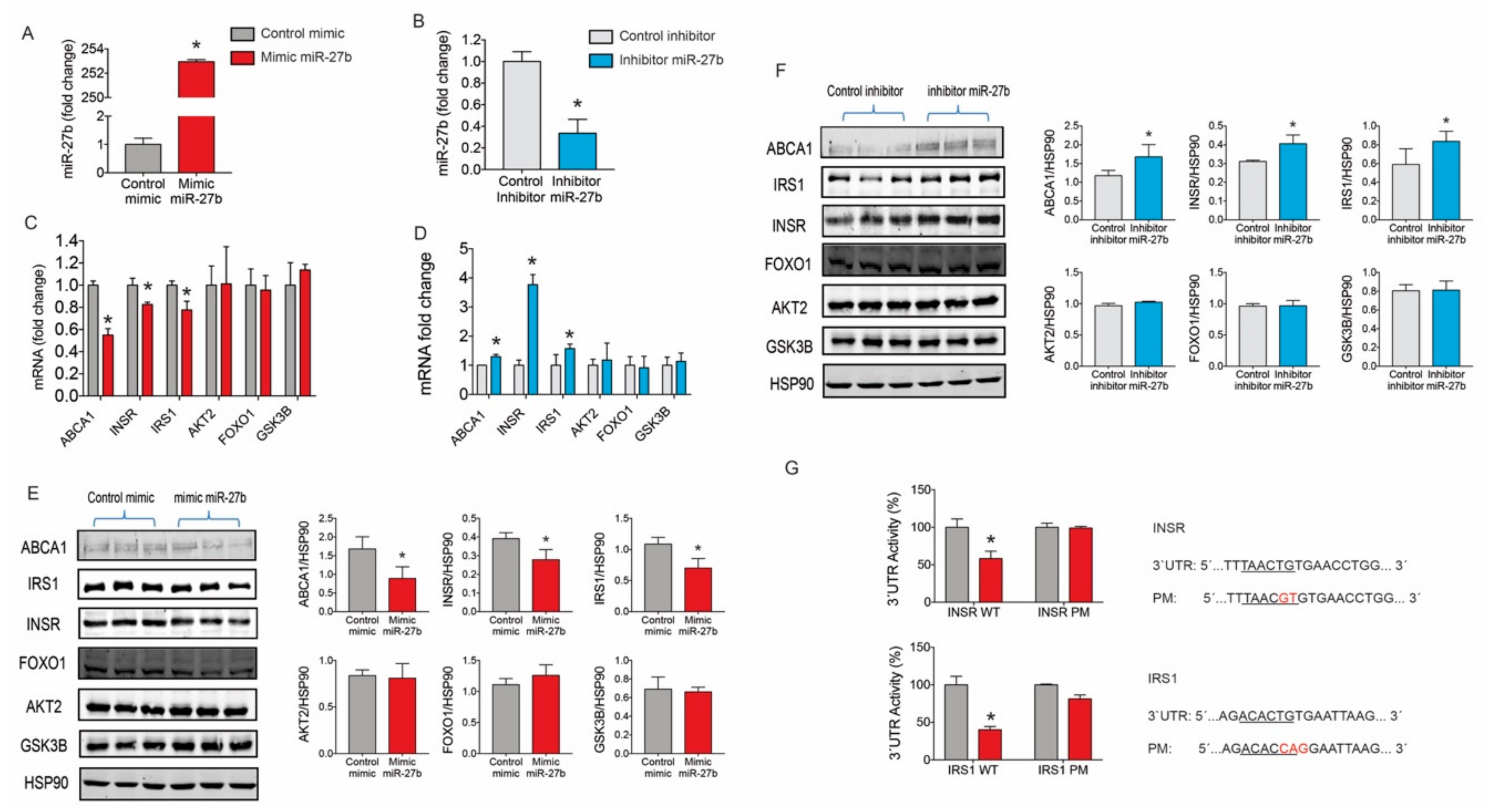
2.2. miR-27b Controls the Expression INSR and IRS1 Expression
2.3. miR-27b Levels Regulate Insulin Signaling in Huh7 Cells
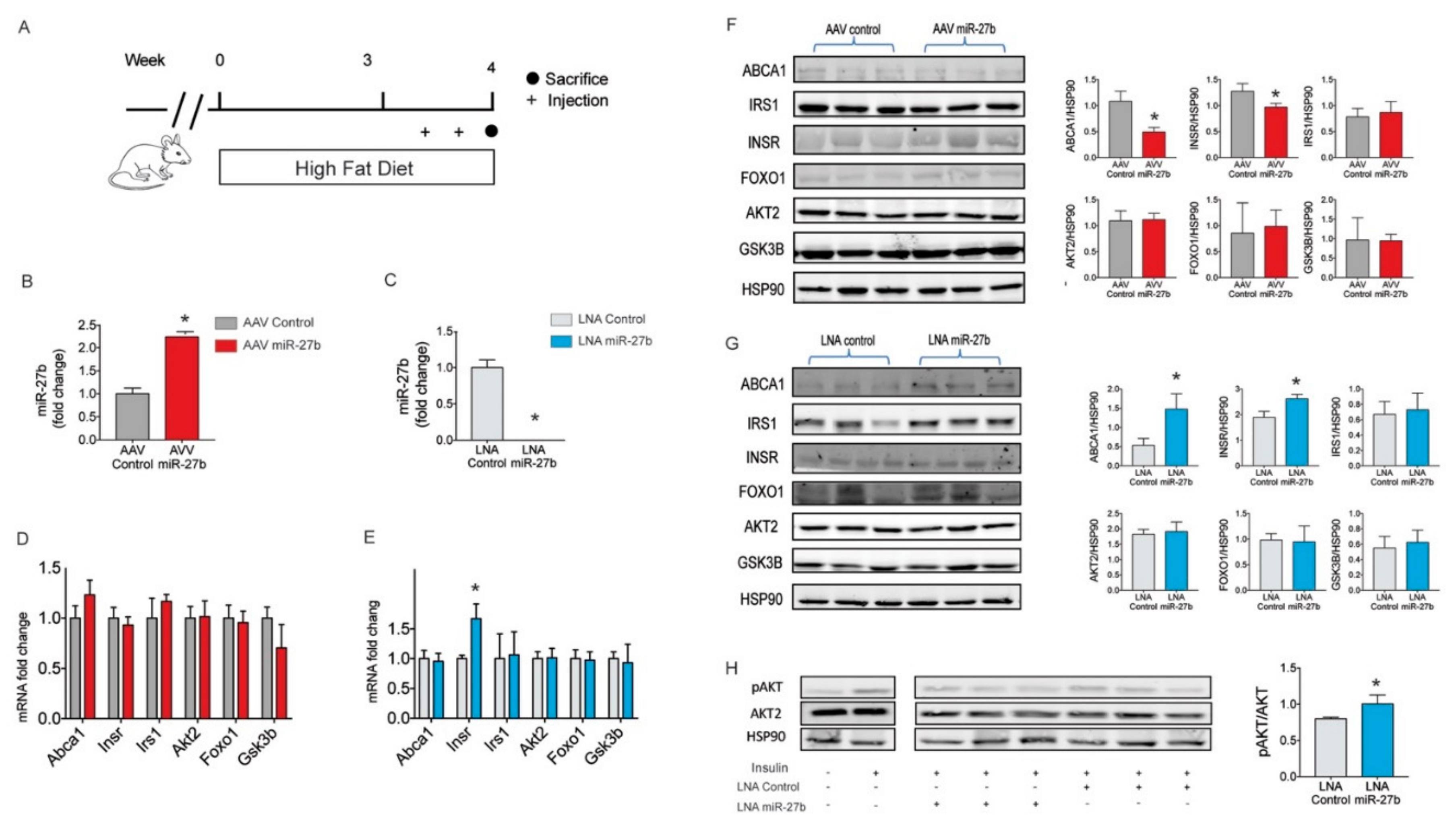
2.4. miR-27b Regulates Hepatic INSR Expression In Vivo
3. Discussion
4. Materials and Methods
4.1. Bioinformatics
4.2. Cell Culture
4.3. In Vitro miRNAs Transfections
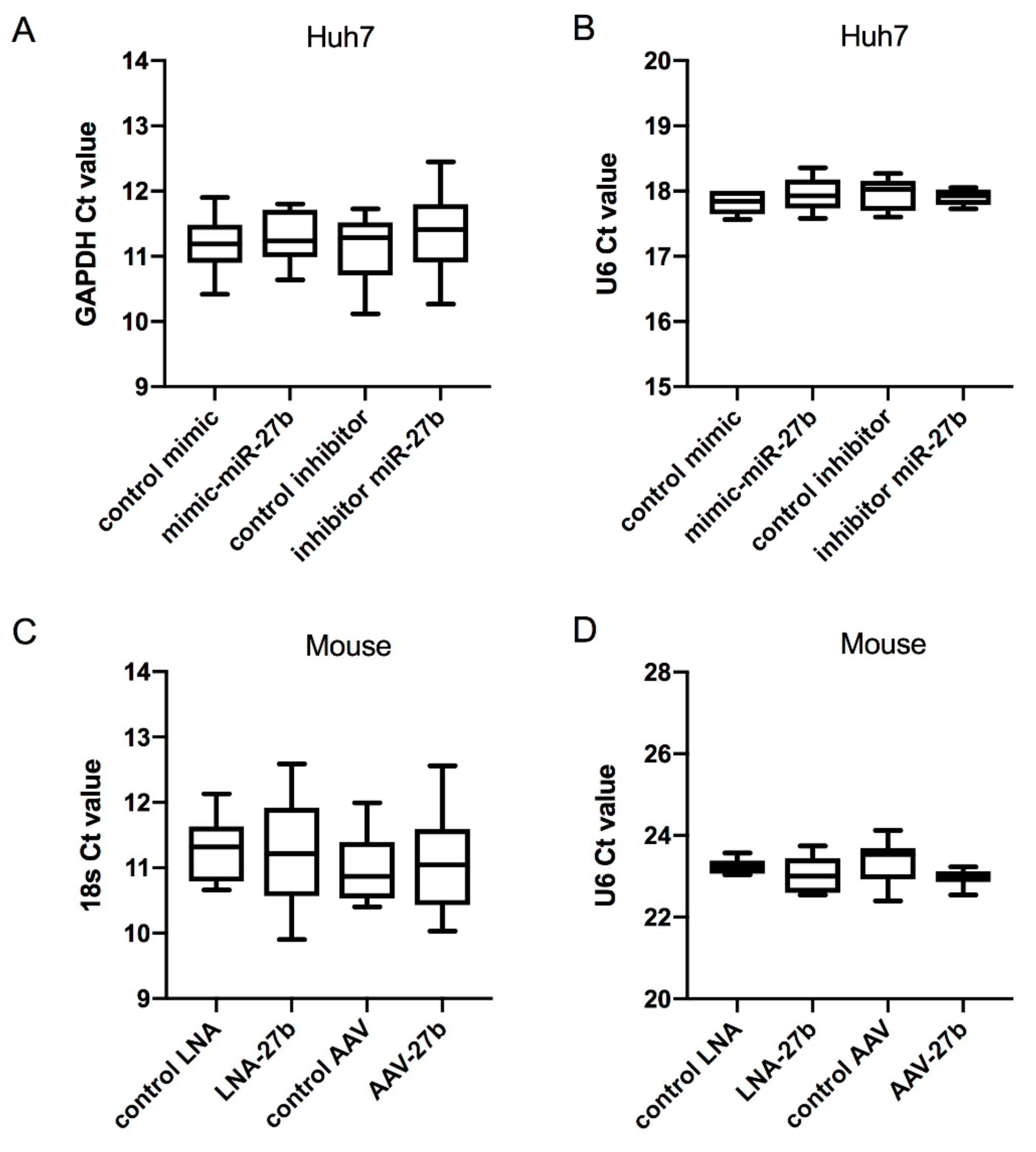
4.4. RNA Isolation and Quantitative PCR Analysis
4.5. Western Blot Analysis
4.6. 3’UTR Luciferase Reporter Assays
4.7. Mice Studies
4.8. miR-27b Overexpression Studies in Mice
4.9. miR-27b Inhibition Studies
4.10. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Gene | Forward Primer Sequence (5′ -> 3′) | Reverse Primer Sequence (5′ -> 3′) |
|---|---|---|
| INSR | AGTTTGAGGACATGGAGAATGTG | ATAGGAACGATCTCTGAACTCCAC |
| IRS1 | ACAAACGCTTCTTCGTACTGC | AGTCAGCCCGCTTGTTGATG |
| GSK3B | GGCAGCATGAAAGTTAGCAGA | GGCGACCAGTTCTCCTGAATC |
| AKT2 | ACCACAGTCATCGAGAGGACC | GGAGCCACACTTGTAGTCCA |
| ABCA1 | ACCCACCCTATGAACAACATGA | GAGTCGGGTAACGGAAACAGG |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| Gene | Antibody | Product Details | Dilution | Host | |
|---|---|---|---|---|---|
| INSR | INSRβ (C18C4) | Novus | NBP1-19192 | 1/1000 | Mouse |
| IRS1 | IRS1 | Novus | NB100-58837 | 1/1000 | Rabbit |
| GSK3B | GSK-3-β (27C10) | Cell Signaling | 9454S | 1/1000 | Rabbit |
| AKT2 | AKT2 (5B5) | Cell Signaling | 2965S | 1/1000 | Rabbit |
| ABCA1 | ABCA1 | Abcam | ab18180 | 1/1000 | Mouse |
| HSP90 | HSP90 | Bd Pharmigen | 610419 | 1/1000 | Mouse |
| Gene | Forward Primer Sequence (5′ -> 3′) | Reverse Primer Sequence (5′ -> 3′) |
|---|---|---|
| Insr | ATGGGCTTCGGGAGAGGAT | CTTCGGGTCTGGTCTTGAACA |
| Irs1 | CGATGGCTTCTCAGACGTG | CAGCCCGCTTGTTGATGTTG |
| Gsk3b | ATGGCAGCAAGGTAACCACAG | TCTCGGTTCTTAAATCGCTTGTC |
| Akt2 | ACGTGGTGAATACATCAAGACC | GGGCCTCTCCTTATACCCAAT |
| Abca1 | GGTTTGGAGATGGTTATACAATAGTTGT | CCCGGAAACGCAAGTCC |
| 18s | TTCCGATAACGAACGAGACTCT | TGGCTGAACGCCACTTGTC |
| Gene | Antibody | Product Details | Dilution | Host | |
|---|---|---|---|---|---|
| AKT | AKT (5B5) | Cell Signaling | 2965S | 1/1000 | Rabbit |
| pAKT | pAKT Ser473 | Cell Signaling | 9271S | 1/1000 | Rabbit |
| HSP90 | HSP90 | Bd Pharmigen | 610419 | 1/1000 | Mouse |
References
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Dyson, P.A. The therapeutics of lifestyle management on obesity. Diabetes Obes. Metab. 2010, 12, 941–946. [Google Scholar] [CrossRef]
- Dirkx, E.; Schwenk, R.W.; Glatz, J.F.C.; Luiken, J.J.F.P.; van Eys, G.J.J.M. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Savastano, S.; Colao, A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J. Gastroenterol. 2010, 16, 4773–4783. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Roszer, T.; Ricote, M. Lipotoxicity in macrophages: Evidence from diseases associated with the metabolic syndrome. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A.; Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis find the latest version: Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation 1997, 95, 1–4. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Kaidanovich, O.; Eldar-Finkelman, H. The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Expert Opin. Ther. Targets 2002, 6, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-J.; Han, H.-S.; Kim, M.-J.; Koo, S.-H. CREB and FoxO1: Two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013, 46, 567–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Christianson, J.; Liu, Z.-X.; Tian, L.; Choi, C.S.; Neschen, S.; Dong, J.; Wood, P.A.; Shulman, G.I. Resistance to High-Fat Diet-Induced Obesity and Insulin Resistance in Mice with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Cell Metab. 2010, 11, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xiao, Y.; Wang, J.; Hou, N.; Cui, W.; Hu, X.; Zeng, F.; Yuan, Y.; Ma, D.; Sun, X.; et al. Dynamic changes in insulin and glucagon during disease progression in rhesus monkeys with obesity-related type 2 diabetes mellitus. Diabetes Obes. Metab. 2019, 21, 1111–1120. [Google Scholar] [CrossRef]
- Paternostro, G.; Camici, P.G.; Lammerstma, A.A.; Marinho, N.; Baliga, R.R.; Kooner, J.S.; Radda, G.K.; Ferrannini, E. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J. Clin. Investig. 1996, 98, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Madiraju, A.K.; Gassaway, B.M.; Marcel, M.; Nasiri, A.R.; Butrico, G.; Marcucci, M.J.; Zhang, D.; Abulizi, A.; Zhang, X.-M.; et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016, 126, 4361–4371. [Google Scholar] [CrossRef] [Green Version]
- Vegter, E.L.; van der Meer, P.; de Windt, L.J.; Pinto, Y.M.; Voors, A.A. MicroRNAs in heart failure: From biomarker to target for therapy. Eur. J. Heart Fail. 2016, 18, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Hernando, C.; Moore, K.J. MicroRNA modulation of cholesterol homeostasis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2378–2382. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Hernando, C.; Suárez, Y.; Rayner, K.J.; Moore, K.J. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 2011, 22, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Aryal, B.; Zhang, X.; Fan, Y.; Price, N.L.; Suárez, Y.; Fernández-Hernando, C. Posttranscriptional regulation of lipid metabolism by non-coding RNAs and RNA binding proteins. Semin. Cell Dev. Biol. 2018, 81, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ren, G.; Zhu, L.; Liu, X.; He, X. The upregulation of miRNA-146a inhibited biological behaviors of ESCC through inhibition of IRS2. Tumor Biol. 2016, 37, 4641–4647. [Google Scholar] [CrossRef] [PubMed]
- Honardoost, M.; Arefian, E.; Soleimani, M.; Soudi, S.; Sarookhani, M.R. Development of Insulin Resistance through Induction of miRNA-135 in C2C12 Cells. Cell J. 2016, 18, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-J.; Park, S.-Y.; Yang, W.-M.; Lee, W. The induction of miR-96 by mitochondrial dysfunction causes impaired glycogen synthesis through translational repression of IRS-1 in SK-Hep1 cells. Biochem. Biophys. Res. Commun. 2013, 434, 503–508. [Google Scholar] [CrossRef]
- Chen, T.; Ding, G.; Jin, Z.; Wagner, M.B.; Yuan, Z. Insulin ameliorates miR-1-induced injury in H9c2 cells under oxidative stress via Akt activation. Mol. Cell. Biochem. 2012, 369, 167–174. [Google Scholar] [CrossRef]
- Melkman-Zehavi, T.; Oren, R.; Kredo-Russo, S.; Shapira, T.; Mandelbaum, A.D.; Rivkin, N.; Nir, T.; Lennox, K.A.; Behlke, M.A.; Dor, Y.; et al. miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. EMBO J. 2011, 30, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Yue, P.; Ma, F.; Yan, H.; Zhang, Y.; Wang, C.; Qiu, D.; Hua, Y.; Li, Y. Interpreting the various associations of MiRNA polymorphisms with susceptibilities of cardiovascular diseases. Medicine (Baltimore) 2018, 97, e10712. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C. Emerging role of MicroRNAs in the regulation of lipid metabolism. Hepatology 2013, 57, 432–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Enokida, H.; Chiyomaru, T.; Kinoshita, T.; Nakagawa, M.; Naya, Y.; Ichikawa, T.; Seki, N. The microRNA-23b/27b/24-1 cluster is a disease progression marker and tumor suppressor in prostate cancer. Oncotarget 2014, 5, 7748–7759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ader, M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2000, 11, 351–356. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. (Lausanne) 2018, 9, 384. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-M.; Jeong, H.-J.; Park, S.-W.; Lee, W. Obesity-induced miR-15b is linked causally to the development of insulin resistance through the repression of the insulin receptor in hepatocytes. Mol. Nutr. Food Res. 2015, 59, 2303–2314. [Google Scholar] [CrossRef]
- Yang, W.-M.; Jeong, H.-J.; Park, S.-Y.; Lee, W. Saturated fatty acid-induced miR-195 impairs insulin signaling and glycogen metabolism in HepG2 cells. FEBS Lett. 2014, 588, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Meng, X.; Che, H.; Shen, N.; Xiao, D.; Song, X.; Liang, M.; Fu, X.; Ju, J.; Li, Y.; et al. Regulation of Insulin Resistance by Multiple MiRNAs via Targeting the GLUT4 Signalling Pathway. Cell. Physiol. Biochem. 2016, 38, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, J.; Hu, X.; Bao, P.; Pan, J.; Chen, Z.; Xian, J. MiR-27b Impairs Adipocyte Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells by Targeting LPL. Cell. Physiol. Biochem. 2018, 47, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Rotllan, N.; Ramírez, C.M.; Aranda, J.F.; Canfrán-Duque, A.; Araldi, E.; Fernández-Hernando, A.; Langhi, C.; de Cabo, R.; Baldán, Á.; et al. miR-27b inhibits LDLR and ABCA1 expression but does not influence plasma and hepatic lipid levels in mice. Atherosclerosis 2015, 243, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirasaki, T.; Honda, M.; Shimakami, T.; Horii, R.; Yamashita, T.; Sakai, Y.; Sakai, A.; Okada, H.; Watanabe, R.; Murakami, S.; et al. MicroRNA-27a Regulates Lipid Metabolism and Inhibits Hepatitis C Virus Replication in Human Hepatoma Cells. J. Virol. 2013, 87, 5270–5286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collares, R.V.A.; Salgado, W.; Da Cunha Tirapelli, D.P.; Dos Santos, J.S. The expression of LEP, LEPR, IGF1 and IL10 in obesity and the relationship with microRNAs. PLoS ONE 2014, 9, e93512. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Shankar, K.; Beg, M.; Rajan, S.; Gupta, A.; Varshney, S.; Kumar, D.; Gupta, S.; Mishra, R.K.; Gaikwad, A.N. Chronic hyperinsulinemia induced miR-27b is linked to adipocyte insulin resistance by targeting insulin receptor. J. Mol. Med. 2018, 96, 315–331. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N.; Kadowaki, T. Imbalanced Insulin Actions in Obesity and Type 2 Diabetes: Key Mouse Models of Insulin Signaling Pathway. Cell Metab. 2017, 25, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Ślusarz, A.; Pulakat, L. The two faces of miR-29. J. Cardiovasc. Med. 2015, 16, 480–490. [Google Scholar] [CrossRef]
- Kato, M.; Castro, N.E.; Natarajan, R. MicroRNAs: Potential mediators and biomarkers of diabetic complications. Free Radic. Biol. Med. 2013, 64, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hong, J.; Cao, Y.; Shi, J.; Gu, W.; Ning, G.; Zhang, Y.; Wang, W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur. J. Endocrinol. 2015, 172, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; DeLemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliasson, L. The small RNA miR-375—a pancreatic islet abundant miRNA with multiple roles in endocrine beta cell function. Mol. Cell. Endocrinol. 2017, 456, 95–101. [Google Scholar] [CrossRef]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell. Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Ramírez, C.M.; Goedeke, L.; Suárez, Y. MicroRNAs in Metabolic Disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, C.M.; Goedeke, L.; Fernández-Hernando, C. “Micromanaging” metabolic syndrome. Cell Cycle 2011, 10, 3249–3252. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, C.M.; Goedeke, L.; Rotllan, N.; Yoon, J.-H.; Cirera-Salinas, D.; Mattison, J.A.; Suarez, Y.; de Cabo, R.; Gorospe, M.; Fernandez-Hernando, C. MicroRNA 33 Regulates Glucose Metabolism. Mol. Cell. Biol. 2013, 33, 2891–2902. [Google Scholar] [CrossRef] [Green Version]
- Rayner, K.J.; Suarez, Y.; Davalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernandez-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito-Vicente, A.; Uribe, K.B.; Rotllan, N.; Ramírez, C.M.; Jebari-Benslaiman, S.; Goedeke, L.; Canfrán-Duque, A.; Galicia-García, U.; Saenz De Urturi, D.; Aspichueta, P.; et al. miR-27b Modulates Insulin Signaling in Hepatocytes by Regulating Insulin Receptor Expression. Int. J. Mol. Sci. 2020, 21, 8675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228675
Benito-Vicente A, Uribe KB, Rotllan N, Ramírez CM, Jebari-Benslaiman S, Goedeke L, Canfrán-Duque A, Galicia-García U, Saenz De Urturi D, Aspichueta P, et al. miR-27b Modulates Insulin Signaling in Hepatocytes by Regulating Insulin Receptor Expression. International Journal of Molecular Sciences. 2020; 21(22):8675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228675
Chicago/Turabian StyleBenito-Vicente, Asier, Kepa B. Uribe, Noemi Rotllan, Cristina M. Ramírez, Shifa Jebari-Benslaiman, Leigh Goedeke, Alberto Canfrán-Duque, Unai Galicia-García, Diego Saenz De Urturi, Patricia Aspichueta, and et al. 2020. "miR-27b Modulates Insulin Signaling in Hepatocytes by Regulating Insulin Receptor Expression" International Journal of Molecular Sciences 21, no. 22: 8675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21228675





