Flavonoid Glycosides from Ziziphus jujuba var. inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis
Abstract
:1. Introduction
2. Results
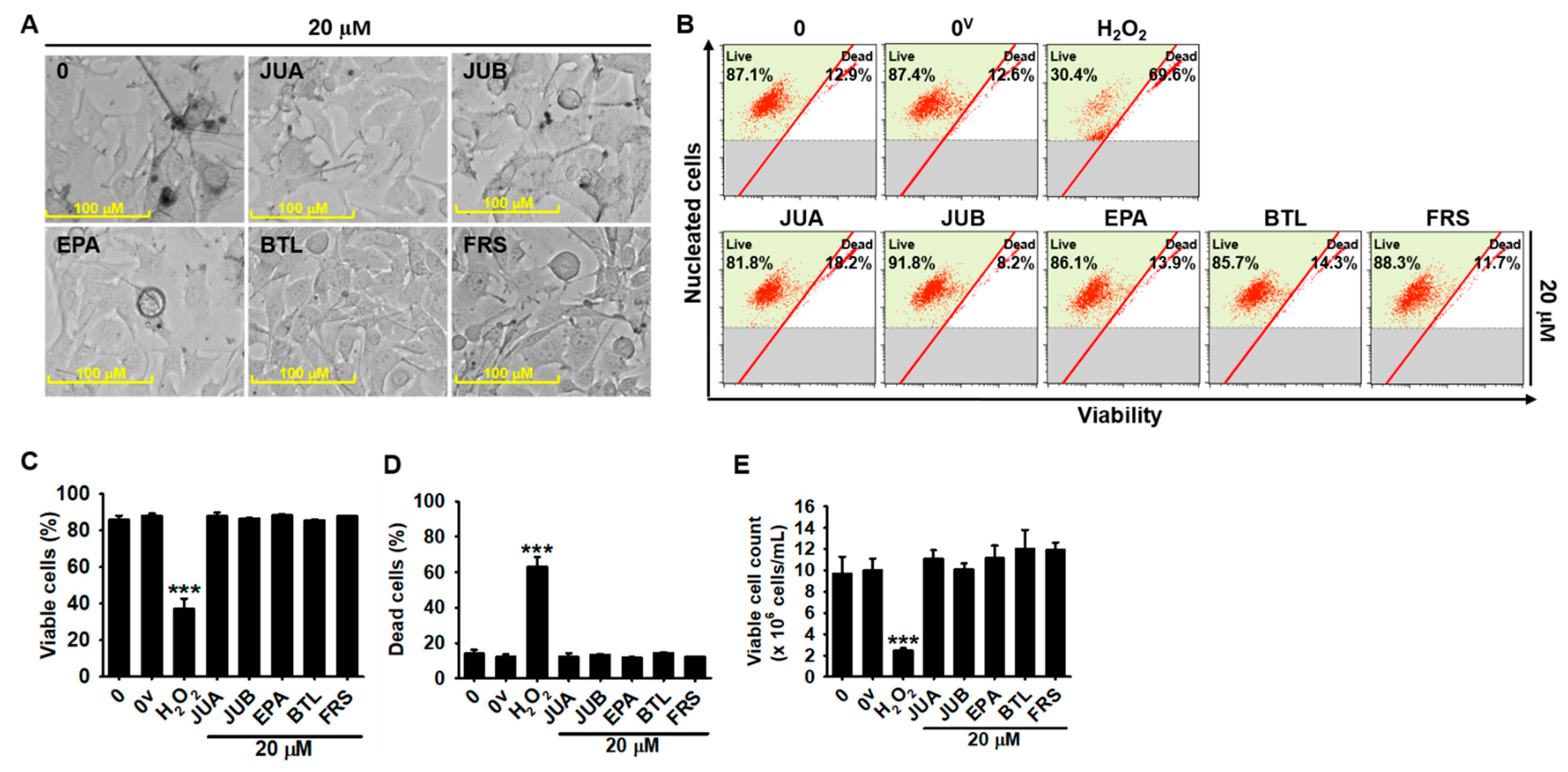
2.1. No Cytotoxic Effects of Flavonoid Glycosides Were Presented in B16F10 Melanoma Cells
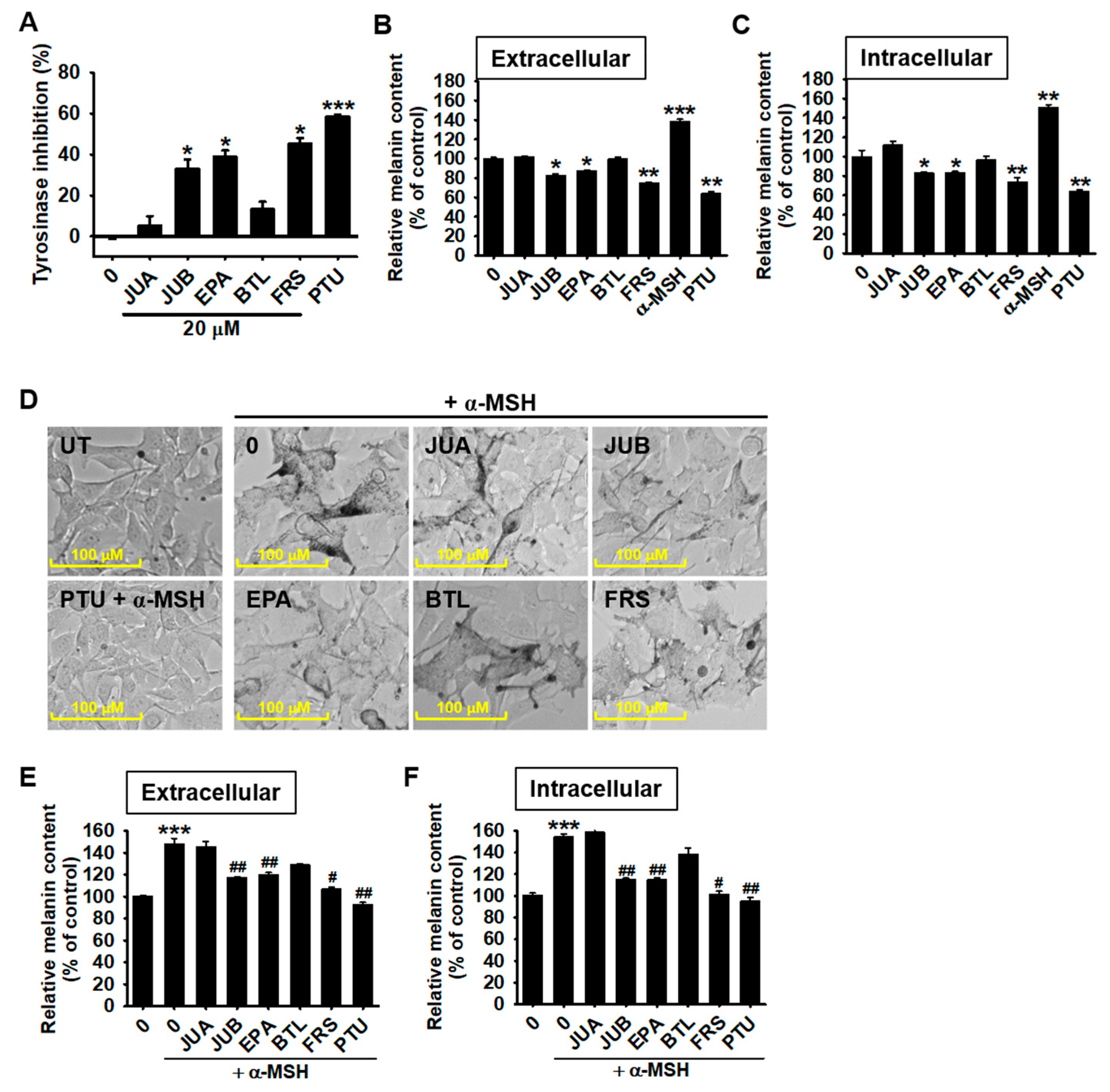
2.2. JUB, EPA, and FRS Inhibit α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells
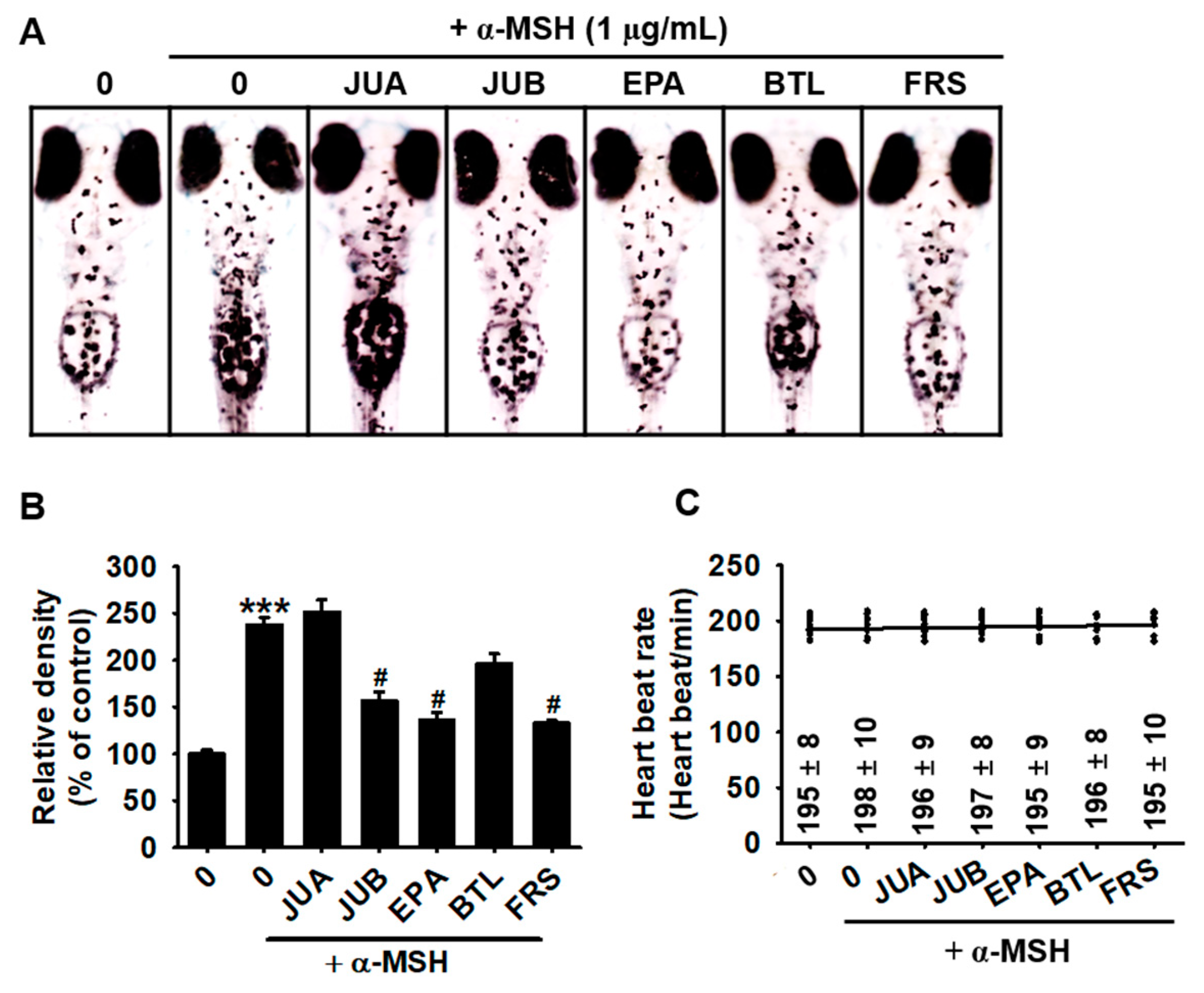
2.3. JUB, EPA, and FRS Prevent α-MSH-Induced Melanogenesis in Zebrafish Larvae
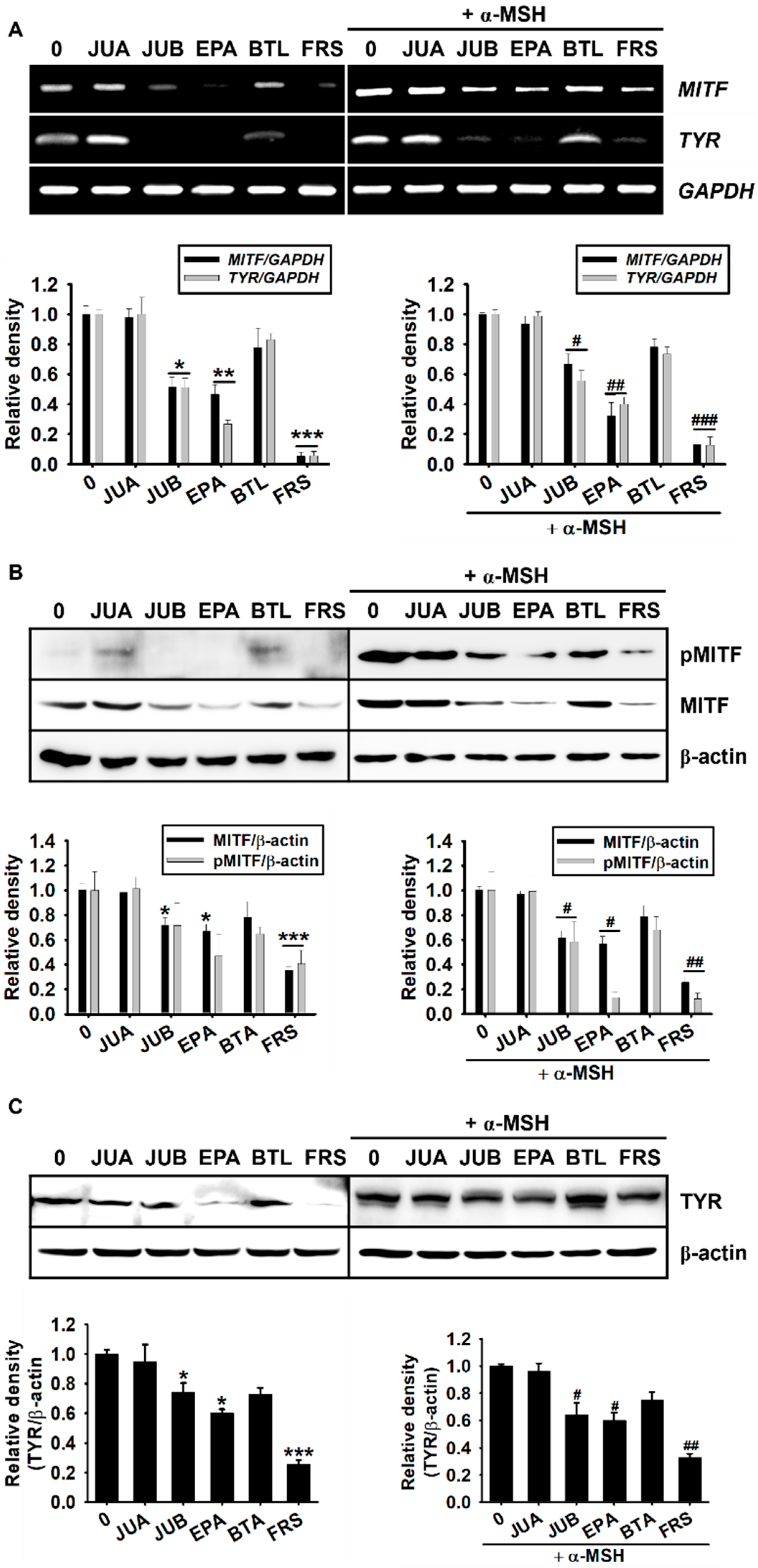
2.4. Anti-Melanogenic Properties of JUB, EPA, and FRS Are Related to Inhibition of MITF and Tyrosinase Expression
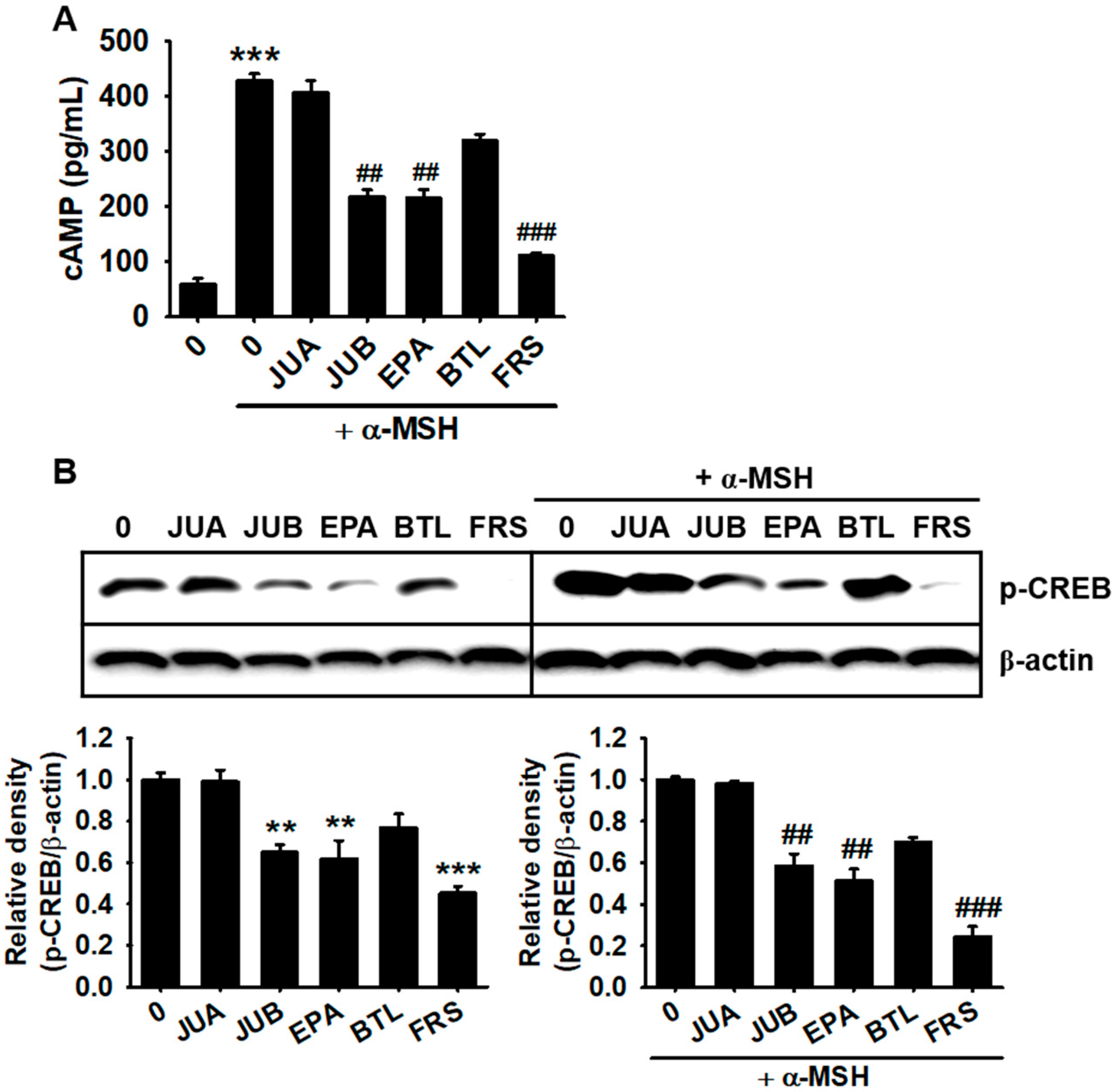
2.5. JUB, EPA, and FRS Inhibit cAMP-Mediated CREB Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability and Morphology
4.4. In Vitro Mushroom Tyrosinase Activity
4.5. Quantification of Extracellular and Intracellular Melanin Content
4.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Protein Extraction and Western Blotting
4.8. Measurement of cAMP
4.9. Maintenance of Zebrafish Embryo and Larvae
4.10. Anti-Melanogenic Effect in Zebrafish Larvae
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.X.; Fukunaga-Kalabis, M.; Herlyn, M. Crosstalk in skin: Melanocytes, keratinocytes, stem cells, and melanoma. J. Cell Commun. Signal. 2016, 10, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Herrling, T.; Jung, K.; Fuchs, J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 1429–1435. [Google Scholar] [CrossRef]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fistarol, S.K.; Itin, P.H. Disorders of pigmentation. J. Deutsch. Dermatol. Ges. 2010, 8, 187–202. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chem. Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Abdoul-Azize, S. Potential benefits of jujube (Zizyphus Lotus L.) bioactive compounds for nutrition and health. J. Nutr. Metab. 2016, 2016, 2867470. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Woo, M.H.; Min, B.S. Anti-inflammatory and cytotoxic activities of constituents isolated from the fruits of Ziziphus jujuba var. inermis Rehder. Fitoterapia 2019, 137, 104261. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Qarah, N.A.S.; Essamadi, A.K.; Moustaid, K.; Nasser, B. Genus Ziziphus: A comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J. Ethnopharmacol. 2020, 259, 112950. [Google Scholar] [CrossRef] [PubMed]
- He, S.-R.; Zhao, C.-B.; Zhang, J.-X.; Wang, J.; Wu, B.; Wu, C.-J. Botanical and traditional uses and phytochemical, pharmacological, pharmacokinetic, and toxicological characteristics of Ziziphi Spinosae Semen: A review. Evid. Based Complement. Alternat. Med. 2020, 2020, 5861821. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Hwang, Y.-H.; Yang, J.-H.; Ma, J.Y.; Lee, B. Spinosin is a flavonoid in the seed of Ziziphus jujuba that prevents skin pigmentation in a human skin model. J. Funct. Foods 2019, 54, 449–456. [Google Scholar] [CrossRef]
- Bang, J.; Zippin, J.H. Cyclic adenosine monophosphate (cAMP) signaling in melanocyte pigmentation and melanomagenesis. Pigment. Cell Melanoma Res. 2021, 34, 28–43. [Google Scholar] [CrossRef] [PubMed]
- BuscÀ, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment. Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Kim, C.-S.; Oh, E.-H.; Lee, M.-K.; Eun, J.-S.; Hong, J.-T.; Oh, K.-W. Methanol extract of Zizyphi Spinosi Semen augments pentobarbital-induced sleep through the modification of GABAergic systems. Nat. Prod. Sci. USA 2012, 18, 67–75. [Google Scholar]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.-A.; Li, Y.; Wang, R.; Yan, H.; Qian, D.; Tang, Y.; Su, S. Comparison of the bioactive components in two seeds of Ziziphus species by different analytical approaches combined with chemometrics. Front. Pharmacol. 2017, 8, 609. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Ahn, J.-B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of Jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, J.; Bian, Y.; Sheng, M.; Liu, B.; Fu, Z.; Zhang, Y.; Yang, B. Jujuboside B reduces vascular tension by increasing Ca2+ influx and activating endothelial nitric oxide synthase. PLoS ONE 2016, 11, e0149386. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.-Y.; Lee, S.Y.; Kang, S.S.; Kim, Y.S. Antitumor activity of uujuboside B and the underlying mechanism via induction of apoptosis and autophagy. J. Nat. Prod. 2014, 77, 370–376. [Google Scholar] [CrossRef]
- Seo, E.J.; Lee, S.Y.; Kang, S.S.; Jung, Y.S. Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytother. Res. 2013, 27, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-M.; Liao, X.; Peng, S.-L.; Ding, L.-S. Chemical constituents from the seeds of Ziziphus jujuba var. spinosa (Bunge) Hu. J. Integr. Plant Biol. 2005, 47, 494–498. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, L.; Qian, K.; Liu, J.; Zhang, L.; Lee, K.-H.; Sun, H. Efficient synthesis and biological evaluation of epiceanothic acid and related compounds. Bioorg. Med. Chem. Lett. 2011, 21, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zhao, X.; Zhang, Y.; Xie, J.; Zhou, A. 6‴-Feruloylspinosin alleviated beta-amyloid induced toxicity by promoting mitophagy in Caenorhabditis elegans (GMC101) and PC12 cells. Sci. Total. Environ. 2020, 715, 136953. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Misrani, A.; Tang, B.-l.; Chen, J.; Yang, L.; Long, C. Jujuboside A prevents sleep loss-induced disturbance of hippocampal neuronal excitability and memory impairment in young APP/PS1 mice. Sci. Rep. 2019, 9, 4512. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Xie, J. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J. Pharm. Biomed. Anal. 2008, 48, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, J.; Chen, K.; Chen, Y. Jujuboside A and zolpidem inhibiting gamma aminobutyric acid type A receptor. J. Biomech. 2007, 40, S556. [Google Scholar] [CrossRef]
- Shin, M.; Lee, B.M.; Kim, O.; Tran, H.N.K.; Lee, S.; Hwangbo, C.; Min, B.S.; Lee, J.H. Triterpenoids from Ziziphus jujuba induce apoptotic cell death in human cancer cells through mitochondrial reactive oxygen species production. Food Funct. 2018, 9, 3895–3905. [Google Scholar] [CrossRef]
- Chen, J.; Tsim, K.W.K. A Review of edible jujube, the Ziziphus jujuba fruit: A heath food supplement for anemia prevalence. Front. Pharmacol. 2020, 11, 593655. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.-H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK–mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhao, B.; Liu, Y.; Wang, R.; Yang, Y.; Yang, L.; Dong, C. MITF-M regulates melanogenesis in mouse melanocytes. J. Dermatol. Sci. 2018, 90, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.; Zhao, G.; Zhang, H.; Guan, D.; Meng, R.; Zhang, Y.; Yang, Q.; Jia, H.; Dou, K.; Liu, C.; et al. MITF-siRNA formulation is a safe and effective therapy for human melasma. Mol. Ther. 2011, 19, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duckworth, H.W.; Coleman, J.E. Physicochemical and kinetic properties of mushroom tyrosinase. J. Biol. Chem. 1970, 245, 1613–1625. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Karunarathne, W.; Park, S.R.; Choi, Y.H.; Park, E.K.; Jin, C.Y.; Yu, H.; Jo, W.S.; Lee, K.T.; Kim, G.Y. GSK-3b-targeting fisetin promotes melanogenesis in B16F10 melanoma cells and zebrafish larvae through b-catenin activation. Int. J. Mol. Sci. 2020, 21, 312. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]

| Gene * | Primer Sequence (5′–3′) | Size | Tm | Cycle No. |
|---|---|---|---|---|
| MITF | F: 5′- CCCGTCTCTGGAAACTTGATCG -3′ | 403 bp | 60 °C | 27 |
| R: 5′- CTGTACTCTGAGCAGCAGGTC -3′ | ||||
| Tyrosinase | F: 5′- GTCGTCACCCTGAAAATCCTAACT -3′ | 110 bp | 60 °C | 27 |
| R: 5′- CATCGCATAAAACCTGATGGC -3′ | ||||
| GAPDH | F: 5′- ACCACAGTCCATGCCATCAC -3′ | 480 bp | 60 °C | 25 |
| R: 5′- CACCACCCTGTTGCTGTAGC -3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molagoda, I.M.N.; Lee, K.-T.; Athapaththu, A.M.G.K.; Choi, Y.-H.; Hwang, J.; Sim, S.-J.; Kang, S.; Kim, G.-Y. Flavonoid Glycosides from Ziziphus jujuba var. inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis. Int. J. Mol. Sci. 2021, 22, 7701. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147701
Molagoda IMN, Lee K-T, Athapaththu AMGK, Choi Y-H, Hwang J, Sim S-J, Kang S, Kim G-Y. Flavonoid Glycosides from Ziziphus jujuba var. inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis. International Journal of Molecular Sciences. 2021; 22(14):7701. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147701
Chicago/Turabian StyleMolagoda, Ilandarage Menu Neelaka, Kyoung-Tae Lee, Athapaththu Mudiyanselage Gihan Kavinda Athapaththu, Yung-Hyun Choi, Jaeyoung Hwang, Su-Jin Sim, Sanghyuck Kang, and Gi-Young Kim. 2021. "Flavonoid Glycosides from Ziziphus jujuba var. inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis" International Journal of Molecular Sciences 22, no. 14: 7701. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147701







