A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction
Abstract
:1. Introduction
2. Results
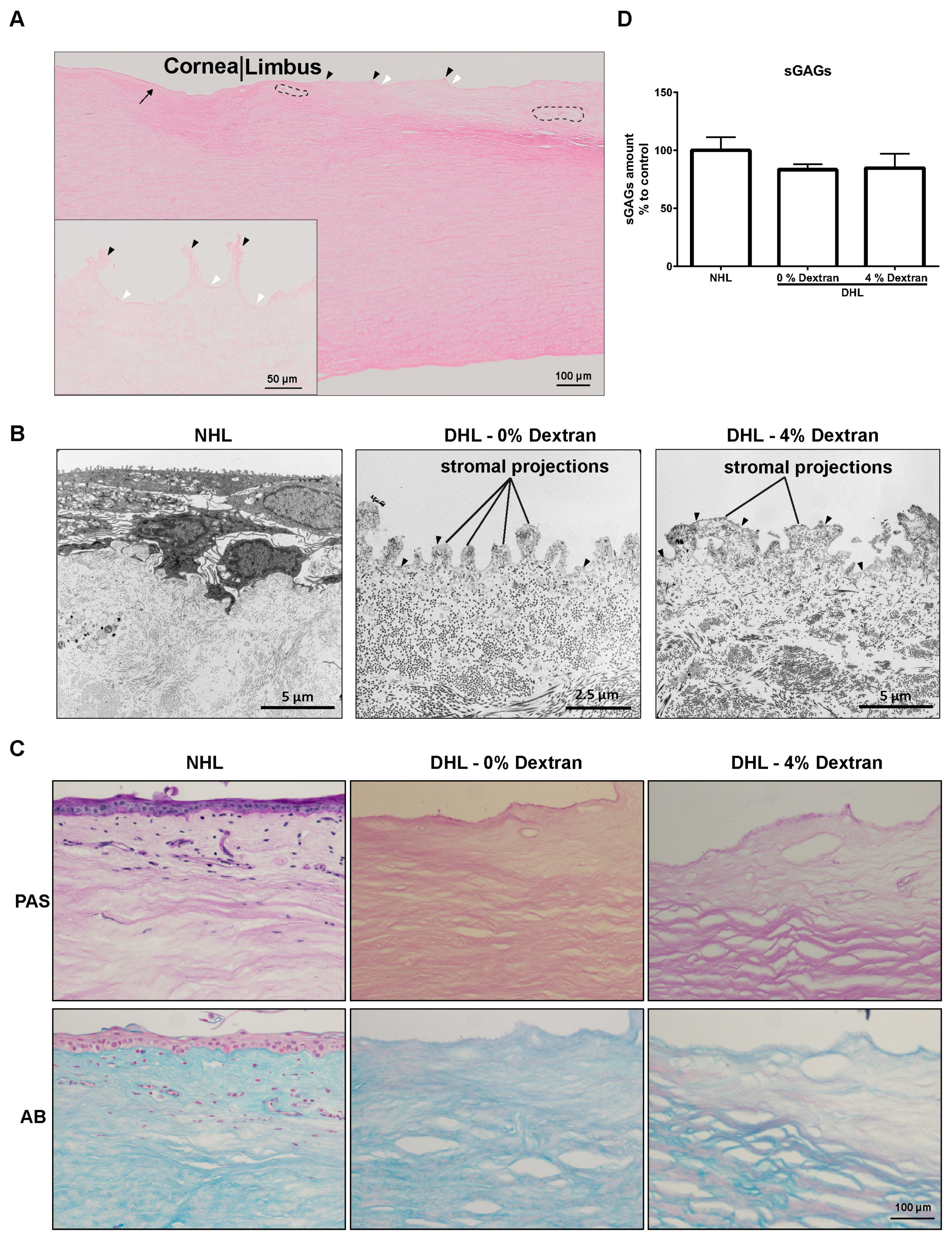
2.1. Efficiency of Decellularization
2.2. Limbal Architecture by Light and Electron Microscopy
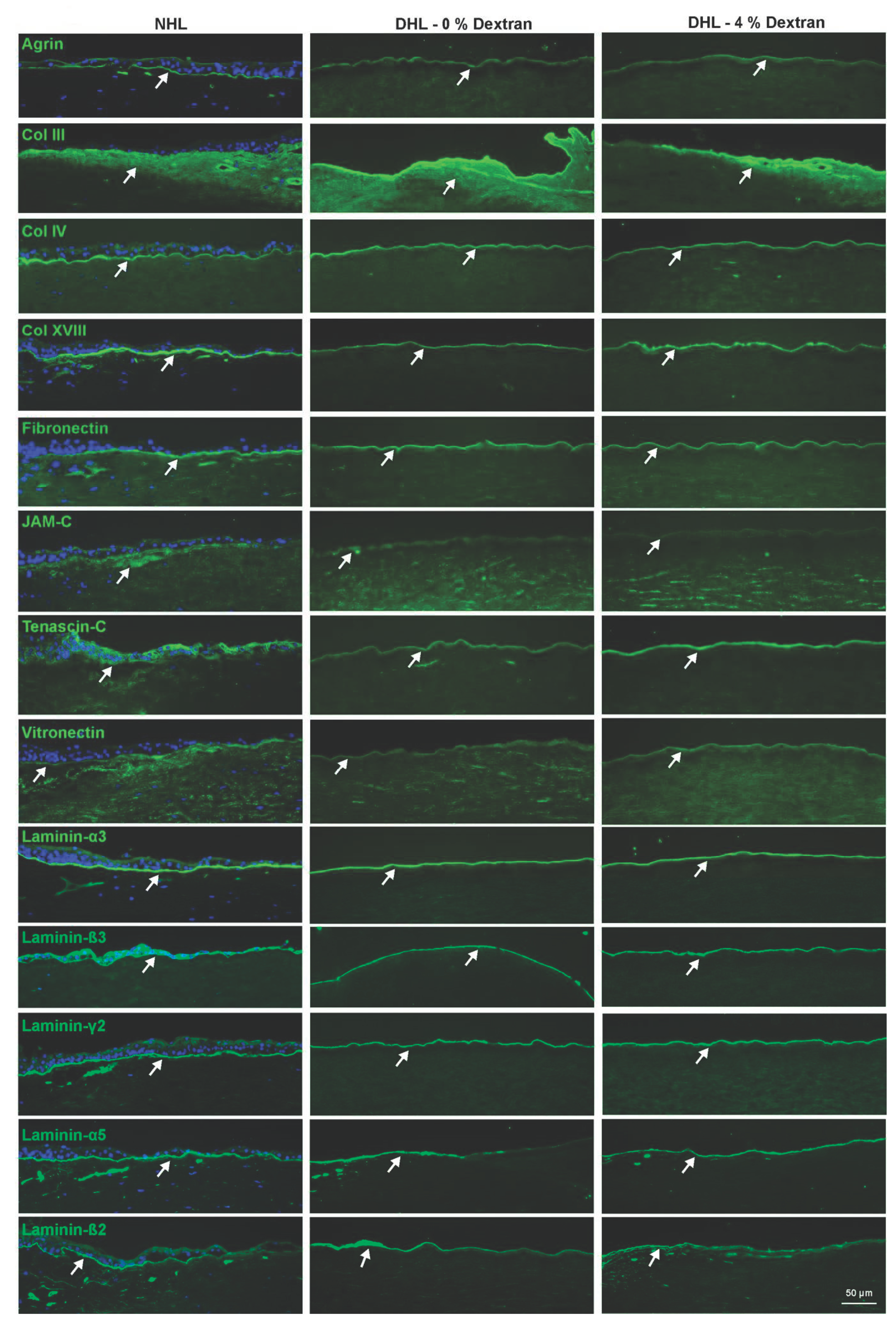
2.3. ECM Components
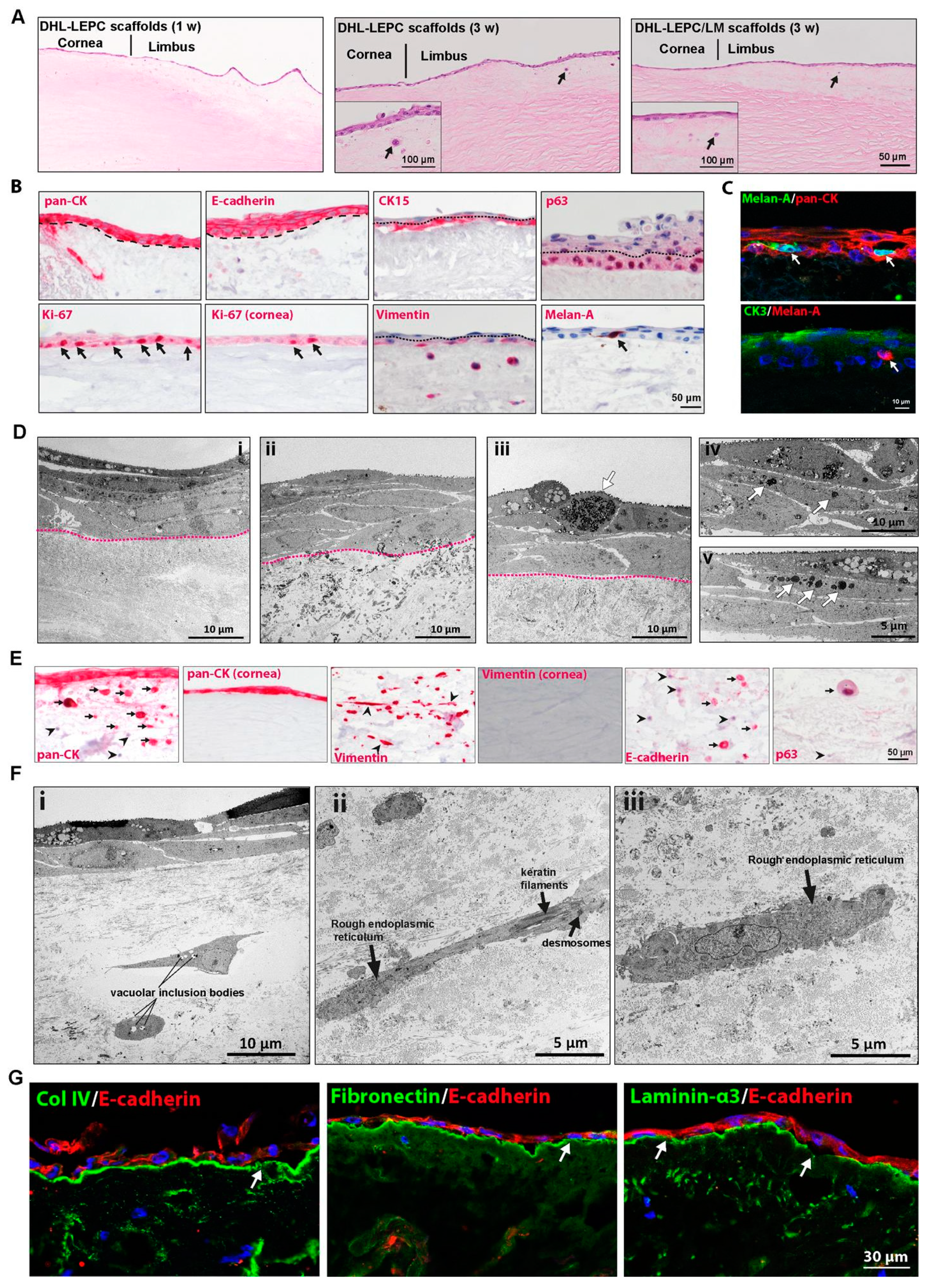
2.4. In Vitro Recellularization
2.5. Ex Vivo Transplantation
3. Discussion
4. Materials and Methods
4.1. Tissue
4.2. Decellularization
4.3. Histology
4.4. Immunostaining of Frozen Sections
4.5. Immunostaining of Paraffin Sections
4.6. Transmission Electron Microscopy
4.7. DNA Content
4.8. Sulfated Glycosaminoglycans
4.9. Cell Culture
4.10. Repopulation of Decellularized Limbal Scaffolds with Cultured Cells
4.11. Ex Vivo Transplantation
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of Slow-Cycling Limbal Epithelial Basal Cells That Can Be Preferentially Stimulated to Proliferate: Implications on Epithelial Stem Cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal Palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar]
- Shortt, A.J.; Secker, G.A.; Munro, P.M.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Characterization of the Limbal Epithelial Stem Cell Niche: Novel Imaging Techniques Permit in Vivo Observation and Targeted Biopsy of Limbal Epithelial Stem Cells. Stem Cells Dayt. Ohio 2007, 25, 1402–1409. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; He, H.; Zhang, S.; Chen, S.-Y. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul. Surf. 2016, 14, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Al, M.; Kyeremeh, G.K.; Saeinasab, M.; Heidari Keshel, S.; Sefat, F. Stem Cell Niche Microenvironment: Review. Bioeng. Basel Switz. 2021, 8, 108. [Google Scholar] [CrossRef]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. The International Limbal Stem Cell Deficiency Working Group Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, T.; Spelsberg, H.; Henke, L.; Kontopoulos, T.; Enczmann, J.; Wernet, P.; Berschick, P.; Sundmacher, R.; Böhringer, D. Long-Term Results of Allogeneic Penetrating Limbo-Keratoplasty in Total Limbal Stem Cell Deficiency. Ophthalmology 2004, 111, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Secker, G.A.; Notara, M.D.; Limb, G.A.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Transplantation of Ex Vivo Cultured Limbal Epithelial Stem Cells: A Review of Techniques and Clinical Results. Surv. Ophthalmol. 2007, 52, 483–502. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-Term Restoration of Damaged Corneal Surfaces with Autologous Cultivated Corneal Epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical Outcomes of Xeno-Free Autologous Cultivated Limbal Epithelial Transplantation: A 10-Year Study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous Limbal Stem Cell Transplantation: A Systematic Review of Clinical Outcomes with Different Surgical Techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.J.-F.; Tsai, R.Y.-N. From Stem Cell Niche Environments to Engineering of Corneal Epithelium Tissue. Jpn. J. Ophthalmol. 2014, 58, 111–119. [Google Scholar] [CrossRef]
- Serna-Ojeda, J.C.; Basu, S.; Vazirani, J.; Garfias, Y.; Sangwan, V.S. Systemic Immunosuppression for Limbal Allograft and Allogenic Limbal Epithelial Cell Transplantation. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 23–32. [Google Scholar] [PubMed]
- Chen, S.-Y.; Mahabole, M.; Tseng, S.C.G. Optimization of Ex Vivo Expansion of Limbal Epithelial Progenitors by Maintaining Native Niche Cells on Denuded Amniotic Membrane. Transl. Vis. Sci. Technol. 2013, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberwein, P.; Reinhard, T. Concise Reviews: The Role of Biomechanics in the Limbal Stem Cell Niche: New Insights for Our Understanding of This Structure. Stem Cells 2015, 33, 916–924. [Google Scholar] [CrossRef]
- Badylak, S.F. The Extracellular Matrix as a Scaffold for Tissue Reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; Ahearne, M. Decellularization and Recellularization of Cornea: Progress towards a Donor Alternative. Methods 2020, 171, 86–96. [Google Scholar] [CrossRef]
- Spaniol, K.; Witt, J.; Mertsch, S.; Borrelli, M.; Geerling, G.; Schrader, S. Generation and Characterisation of Decellularised Human Corneal Limbus. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2018, 256, 547–557. [Google Scholar] [CrossRef]
- Shafiq, M.A.; Milani, B.Y.; Djalilian, A.R. In Vivo Evaluation of a Decellularized Limbal Graft for Limbal Reconstruction. Int. J. Tissue Eng. 2014, 2014, e754245. [Google Scholar] [CrossRef]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.J.T.; Djalilian, A.R. Decellularized Human Cornea for Reconstructing the Corneal Epithelium and Anterior Stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar] [CrossRef]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Corneal Decellularization: A Method of Recycling Unsuitable Donor Tissue for Clinical Translation? Curr. Eye Res. 2016, 41, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Polisetti, N.; Schmid, A.; Schlötzer-Schrehardt, U.; Maier, P.; Lang, S.J.; Steinberg, T.; Schlunck, G.; Reinhard, T. A Decellularized Human Corneal Scaffold for Anterior Corneal Surface Reconstruction. Sci. Rep. 2021, 11, 2992. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of Extracellular Matrix Components in the Limbal Epithelial Stem Cell Compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Swinehart, I.T.; Badylak, S.F. Extracellular Matrix Bioscaffolds in Tissue Remodeling and Morphogenesis. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polisetti, N.; Gießl, A.; Li, S.; Sorokin, L.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511-E8 Promotes Efficient in Vitro Expansion of Human Limbal Melanocytes. Sci. Rep. 2020, 10, 11074. [Google Scholar] [CrossRef] [PubMed]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal Melanocytes Support Limbal Epithelial Stem Cells in 2D and 3D Microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hu, F.-R.; Wang, I.-J.; Hou, Y.-C.; Chen, W.-L. Migration of Limbal Melanocytes onto the Central Cornea after Ocular Surface Reconstruction: An in Vivo Confocal Microscopic Case Report. Cornea 2010, 29, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Gießl, A.; Zenkel, M.; Heger, L.; Dudziak, D.; Naschberger, E.; Stich, L.; Steinkasserer, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Melanocytes as Emerging Key Players in Niche Regulation of Limbal Epithelial Stem Cells. Ocul. Surf. 2021, 22, 172–189. [Google Scholar] [CrossRef]
- Borderie, V.M.; Ghoubay, D.; Georgeon, C.; Borderie, M.; de Sousa, C.; Legendre, A.; Rouard, H. Long-Term Results of Cultured Limbal Stem Cell Versus Limbal Tissue Transplantation in Stage III Limbal Deficiency. Stem Cells Transl. Med. 2019, 8, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef]
- Behaegel, J.; Zakaria, N.; Tassignon, M.-J.; Leysen, I.; Bock, F.; Koppen, C.; Ní Dhubhghaill, S. Short- and Long-Term Results of Xenogeneic-Free Cultivated Autologous and Allogeneic Limbal Epithelial Stem Cell Transplantations. Cornea 2019, 38, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized Scaffolds for Tissue Engineering: Current Status and Future Perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef] [Green Version]
- Isidan, A.; Liu, S.; Chen, A.M.; Zhang, W.; Li, P.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Comparison of Porcine Corneal Decellularization Methods and Importance of Preserving Corneal Limbus through Decellularization. PLoS ONE 2021, 16, e0243682. [Google Scholar] [CrossRef]
- Pellegrini, G.; Rama, P.; Matuska, S.; Lambiase, A.; Bonini, S.; Pocobelli, A.; Colabelli, R.G.; Spadea, L.; Fasciani, R.; Balestrazzi, E.; et al. Biological Parameters Determining the Clinical Outcome of Autologous Cultures of Limbal Stem Cells. Regen. Med. 2013, 8, 553–567. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H. Growth Factors in the Anterior Segment: Role in Tissue Maintenance, Wound Healing and Ocular Pathology. Exp. Eye Res. 2004, 79, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Zenkel, M.; Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 2016, 34, 203–219. [Google Scholar] [CrossRef]
- Hou, L.; Fu, W.; Liu, Y.; Wang, Q.; Wang, L.; Huang, Y. Agrin Promotes Limbal Stem Cell Proliferation and Corneal Wound Healing Through Hippo-Yap Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The Corneal Epithelial Basement Membrane: Structure, Function, and Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Fraemohs, L.; Dejana, E. The Role of Junctional Adhesion Molecules in Vascular Inflammation. Nat. Rev. Immunol. 2007, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Sorokin, L.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511 and -521-Based Matrices for Efficient Ex Vivo-Expansion of Human Limbal Epithelial Progenitor Cells. Sci. Rep. 2017, 7, 5152. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, Q.; Wang, J.; Zhao, X.; Duan, H.; Wang, Y.; Liu, T.; Xie, L. Characterization of the Corneal Surface in Limbal Stem Cell Deficiency and after Transplantation of Cultured Allogeneic Limbal Epithelial Cells. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1765–1777. [Google Scholar] [CrossRef]
- Eslani, M.; Haq, Z.; Movahedan, A.; Moss, A.; Baradaran-Rafii, A.; Mogilishetty, G.; Holland, E.J.; Djalilian, A.R. Late Acute Rejection after Allograft Limbal Stem Cell Transplantation: Evidence for Long-Term Donor Survival. Cornea 2017, 36, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Identification and Characterization of Limbal Stem Cells—Science Direct. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0014483505000801?via%3Dihub (accessed on 8 July 2021).
- Mazzotta, C.; Balestrazzi, A.; Baiocchi, S.; Traversi, C.; Caporossi, A. Stromal Haze after Combined Riboflavin-UVA Corneal Collagen Cross-Linking in Keratoconus: In Vivo Confocal Microscopic Evaluation. Clin. Exp. Ophthalmol. 2007, 35, 580–582. [Google Scholar] [CrossRef]
- Henkind, P. Migration of Limbal Melanocytes. Nature 1967, 214, 1349–1351. [Google Scholar] [CrossRef]
- Gupta, N.; Farooqui, J.H.; Dziasko, M.A.; Daniels, J.T.; Mathur, U.; Sangwan, V.S. Reappearance of Limbal Pigmentation Post-Simple Limbal Epithelial Transplant. Indian J. Ophthalmol. 2020, 68, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Espana, E.M.; He, H.; Li, W.; Liu, C.-Y.; Tseng, S.C.G. Intrastromal Invasion by Limbal Epithelial Cells Is Mediated by Epithelial-Mesenchymal Transition Activated by Air Exposure. Am. J. Pathol. 2005, 167, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.K.; He, H.; Tseng, S.C.G. Epidermal Differentiation and Loss of Clonal Growth Potential of Human Limbal Basal Epithelial Progenitor Cells during Intrastromal Invasion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; He, H.; Kuo, C.-L.; Tseng, S.C.G. The Fate of Limbal Epithelial Progenitor Cells during Explant Culture on Intact Amniotic Membrane. Investig. Ophthalmol. Vis. Sci. 2007, 48, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, C.; Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Lapp, T.; Horres, R.; Agostini, H.; Martin, G.; Reinhard, T.; et al. Expression of the COVID-19 Receptor ACE2 in the Human Conjunctiva. J. Med. Virol. 2020, 92, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Bachmann, B.O.; Laaser, K.; Cursiefen, C.; Kruse, F.E. Characterization of the Cleavage Plane in DESCemet’s Membrane Endothelial Keratoplasty. Ophthalmology 2011, 118, 1950–1957. [Google Scholar] [CrossRef]


| Corneal Samples | Number | Donor Age (years) * | Cultivation Duration (days) * |
|---|---|---|---|
| Total | 136 | 69.1.2 ± 12.7 (25–96) | 39.8 ± 11.2 (8–79) |
| 70 | 68.4 ± 11.2 (44–88) | 38.0 ± 7.6 (22–59) |
| 37 | 79.8 ± 12.6 (51–96) | 41.3 ± 9.7 (8–79) |
| 29 | 59.1 ± 14.5 (25–84) | 40.3 ± 16.4 (26–73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810067
Polisetti N, Roschinski B, Schlötzer-Schrehardt U, Maier P, Schlunck G, Reinhard T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. International Journal of Molecular Sciences. 2021; 22(18):10067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810067
Chicago/Turabian StylePolisetti, Naresh, Benjamin Roschinski, Ursula Schlötzer-Schrehardt, Philip Maier, Günther Schlunck, and Thomas Reinhard. 2021. "A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction" International Journal of Molecular Sciences 22, no. 18: 10067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810067






