Burn Injury Induces Proinflammatory Plasma Extracellular Vesicles That Associate with Length of Hospital Stay in Women: CRP and SAA1 as Potential Prognostic Indicators
Abstract
:1. Introduction
2. Results
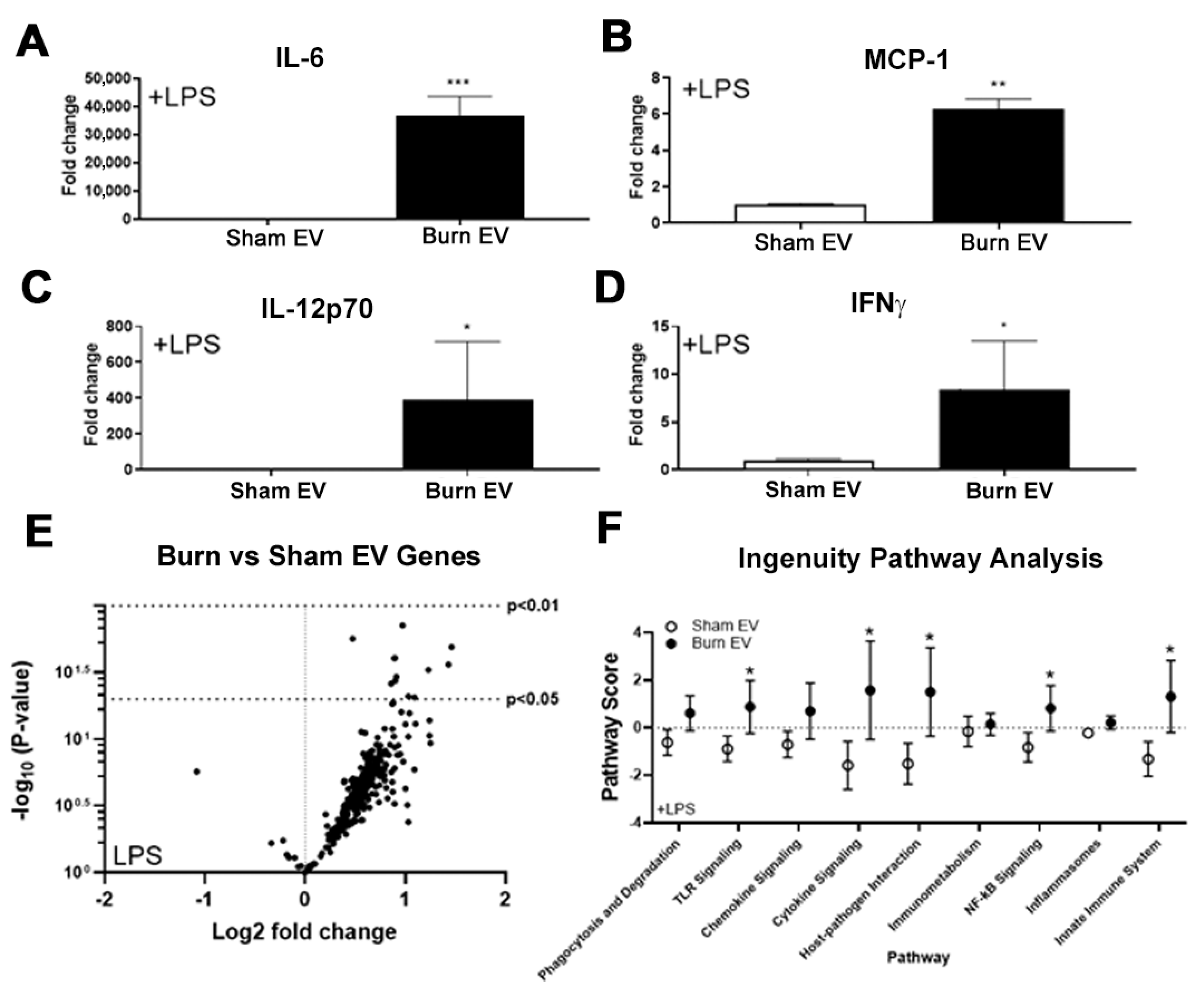
2.1. Plasma EVs from Burn Mice Reproduce Burn-Associated Immune Dysfunction in Cultured Splenic Macrophages
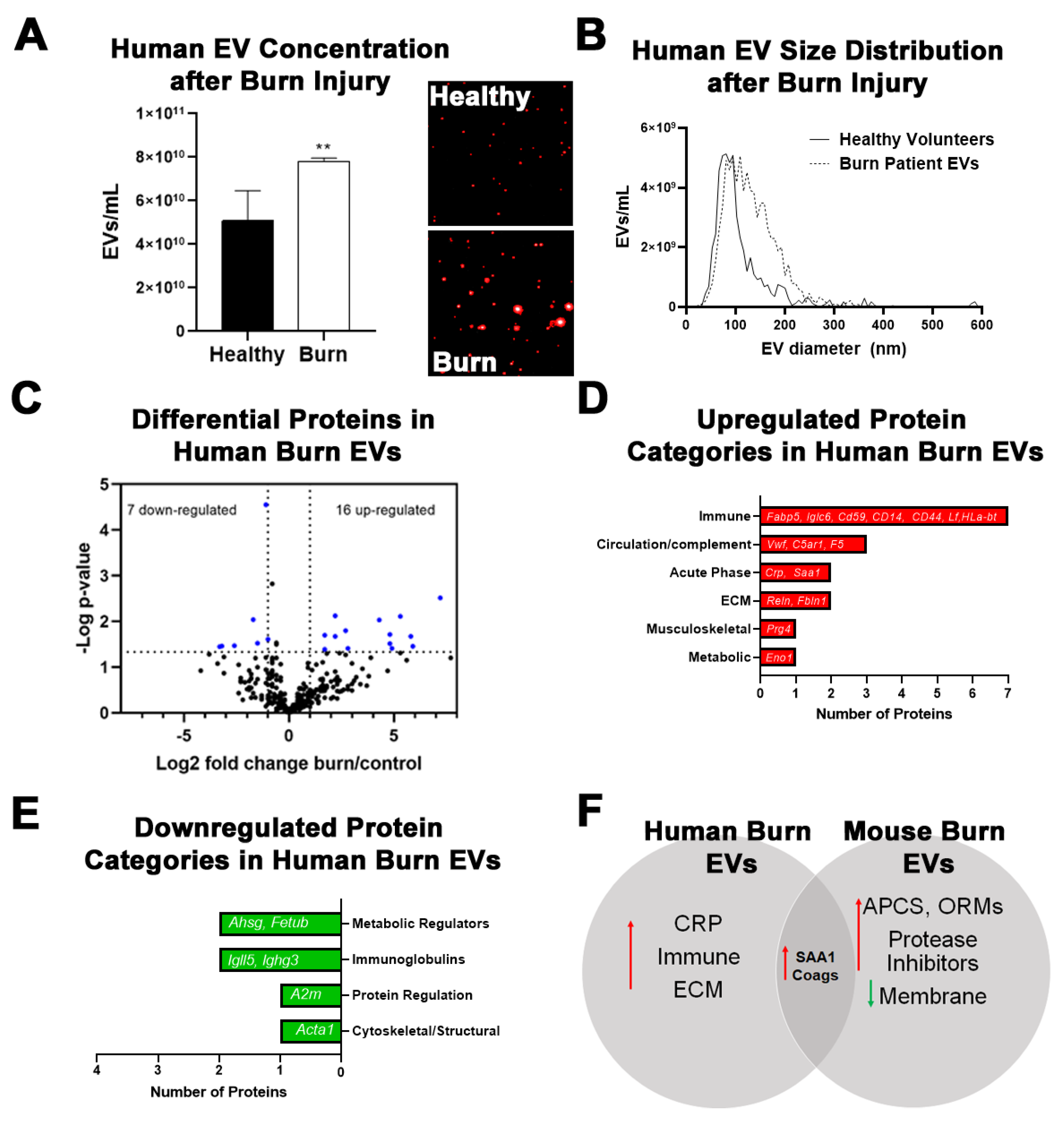
2.2. Large Burn Injury in Mice Alters Plasma EV Proteomic Profile
2.3. Burn Injury in Humans Alters Proteomic Profile of Plasma EVs with Some Similarities to Mice
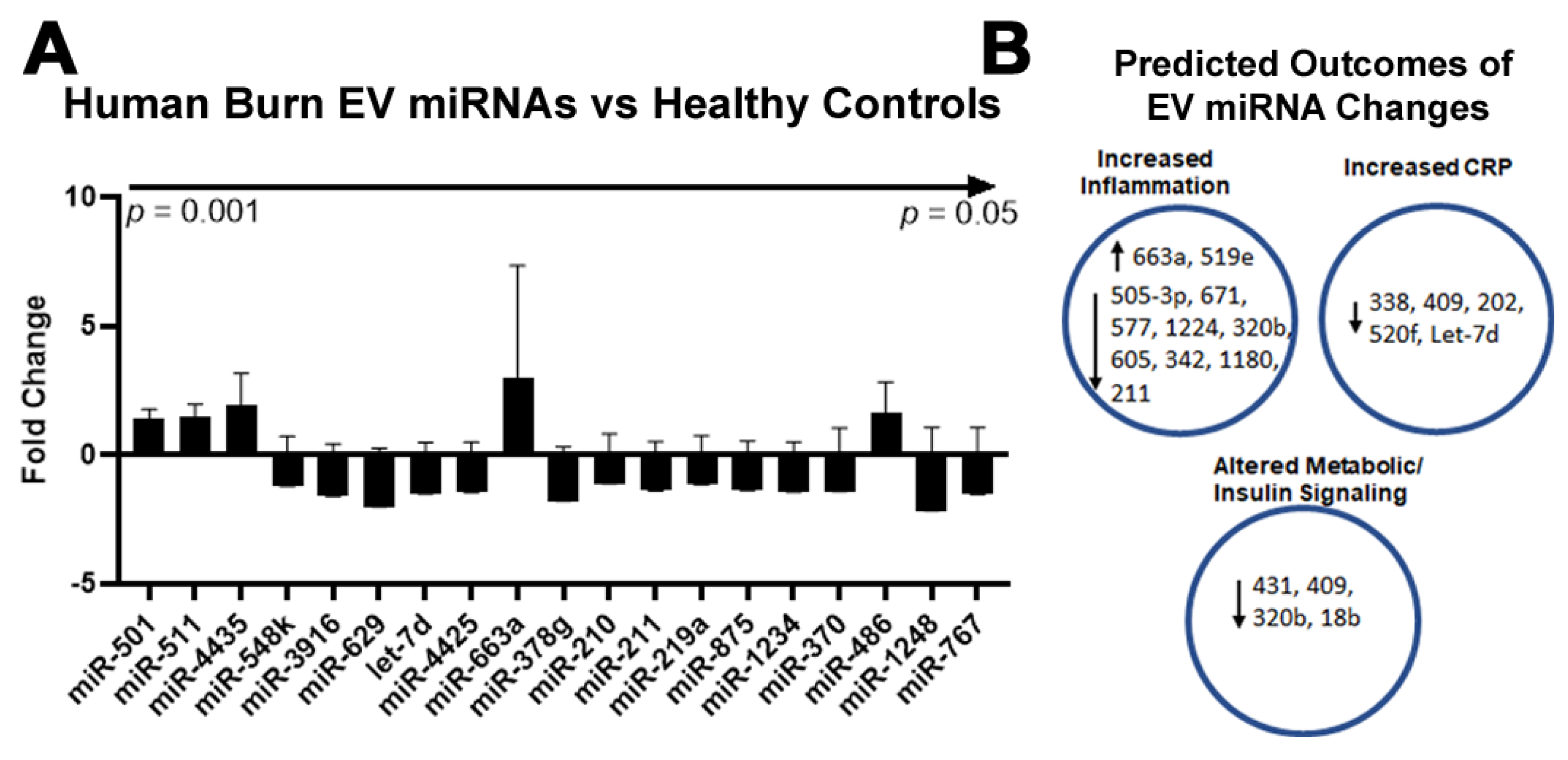
2.4. Burn Injury in Humans Alters miRNAs in Plasma EVs
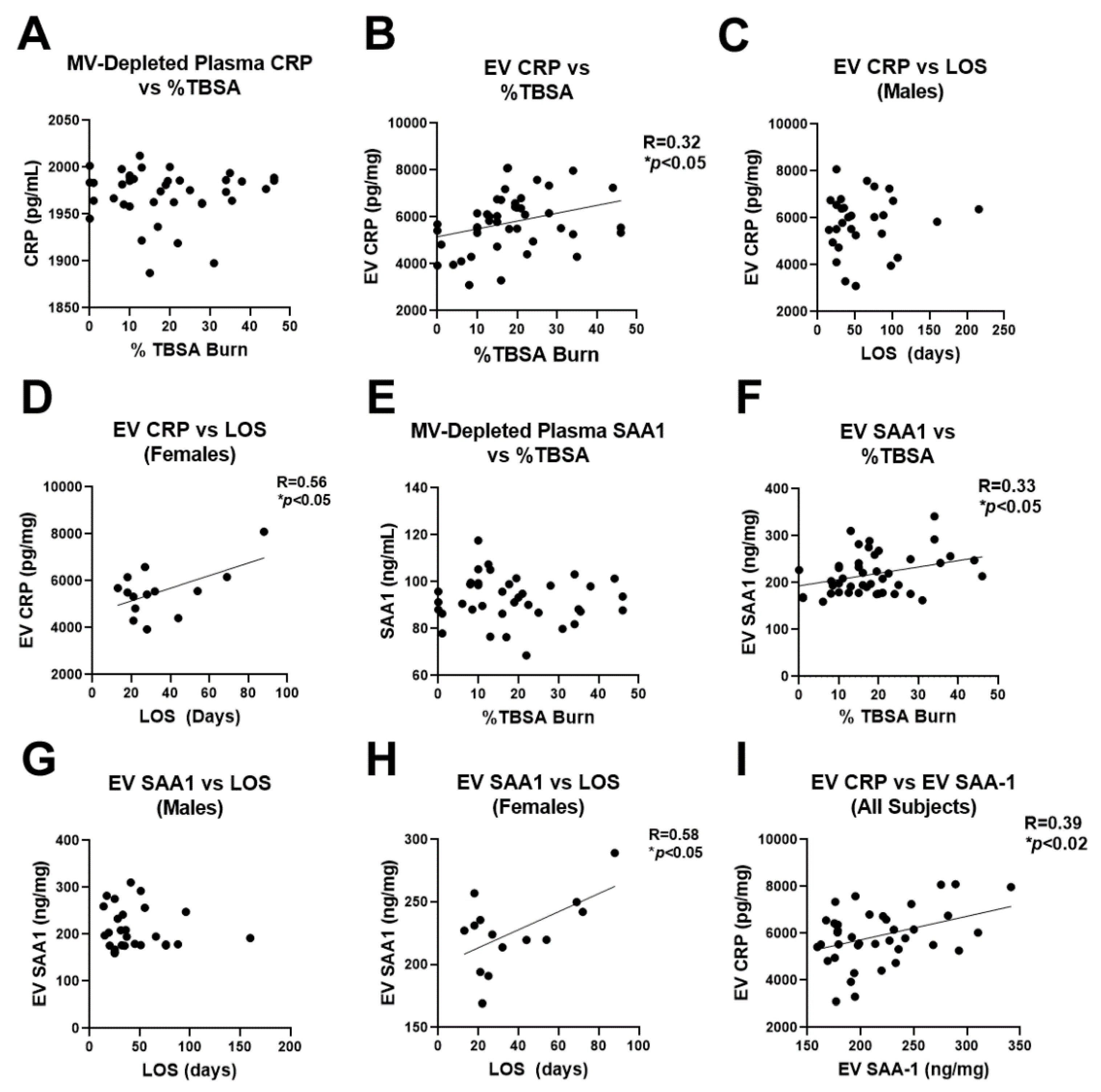
2.5. Plasma EV Number Correlates with Disease Severity
2.6. Human Burn Patients Have Increased Levels of CRP and SAA1 in Plasma EVs That Predict Hospital Length of Stay
3. Discussion
4. Materials and Methods
4.1. Mouse 20% TBSA Thermal Injury
4.2. EV Isolation, Quantification, and Sizing
4.3. Splenic Macrophage Culture and EV-Stimulation
4.4. Measurement of Cytokines and Chemokines in Burn- and Sham-EV Treated Splenic Macrophages
4.5. Macrophage Immune Gene Detection and Quantification by NanoString
4.6. Recruitment of Human Burn Patients and Collection of Human Plasma and Multiplexed NanoString Measurement of miRNAs in Human EVs
4.7. Unbiased Proteomic Assessment of EVs from Mice and Humans and Using LC-MS/MS
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peck, M.; Molnar, J.; Swart, D. A global plan for burn prevention and care. Bull. World Health Organ. 2009, 87, 802–803. [Google Scholar] [CrossRef]
- Miller, S.F.; Bessey, P.; Lentz, C.W.; Jeng, J.C.; Schurr, M.; Browning, S.; Committee, A.N. National burn repository 2007 report: A synopsis of the 2007 call for data. J. Burn Care Res. 2008, 29, 862–870; discussion 871. [Google Scholar] [CrossRef]
- ABA National Burn Repository. Burn Incidence and Treatment in the United States: 2016. Available online: http://ameriburn.org/resources_factsheet.php (accessed on 1 September 2021).
- Maile, R.; Jones, S.; Pan, Y.; Zhou, H.; Jaspers, I.; Peden, D.B.; Cairns, B.A.; Noah, T.L. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L855–L860. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Maile, R.; Jones, S.W.; Cairns, B.A.; Crews, F.T. HMGB1/IL-1beta complexes in plasma microvesicles modulate immune responses to burn injury. PLoS ONE 2018, 13, e0195335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, M.L.; Mahung, C.; Wallet, S.M.; Barnett, A.; Cairns, B.A.; Coleman, L.G., Jr.; Maile, R. Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function. J. Leukoc. Biol. 2021, 1, 17. [Google Scholar]
- Cairns, B.A.; Barnes, C.M.; Mlot, S.; Meyer, A.A.; Maile, R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. J. Trauma Acute Care Surg. 2008, 64, 1069–1078. [Google Scholar] [CrossRef]
- Moore, C.B.; Medina, M.A.; van Deventer, H.W.; O’Connor, B.P.; Cameron, S.; Taxman, D.J.; Maile, R.; Ting, J.P.; Cairns, B.A. Downregulation of immune signaling genes in patients with large surface burn injury. J. Burn Care Res. 2007, 28, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.D.; Karavitis, J.; Kovacs, E.J. Sex differences and estrogen modulation of the cellular immune response after injury. Cell. Immunol. 2008, 252, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Raeven, P.; Zipperle, J.; Drechsler, S. Extracellular Vesicles as Markers and Mediators in Sepsis. Theranostics 2018, 8, 3348–3365. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dea, K.P.; Porter, J.R.; Tirlapur, N.; Katbeh, U.; Singh, S.; Handy, J.M.; Takata, M. Circulating Microvesicles Are Elevated Acutely following Major Burns Injury and Associated with Clinical Severity. PLoS ONE 2016, 11, e0167801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Fahy, K.E.; Awoyemi, A.A.; Thapa, P.; Kelly, L.E.; Chen, J.; Bihl, J.C.; Cool, D.R.; Chen, Y.; Rapp, C.M.; et al. Thermal Burn Injury Generates Bioactive Microvesicles: Evidence for a Novel Transport Mechanism for the Lipid Mediator Platelet-Activating Factor (PAF) That Involves Subcellular Particles and the PAF Receptor. J. Immunol. 2020, 205, 193–201. [Google Scholar] [CrossRef]
- Eitas, T.K.; Stepp, W.H.; Sjeklocha, L.; Long, C.V.; Riley, C.; Callahan, J.; Sanchez, Y.; Gough, P.; Knowlin, L.; van Duin, D.; et al. Differential regulation of innate immune cytokine production through pharmacological activation of Nuclear Factor-Erythroid-2-Related Factor 2 (NRF2) in burn patient immune cells and monocytes. PLoS ONE 2017, 12, e0184164. [Google Scholar] [CrossRef]
- Kartchner, L.B.; Gode, C.J.; Dunn, J.L.M.; Glenn, L.I.; Duncan, D.N.; Wolfgang, M.C.; Cairns, B.A.; Maile, R. One-hit wonder: Late after burn injury, granulocytes can clear one bacterial infection but cannot control a subsequent infection. Burns 2019, 45, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.; Maile, R.; Barnes, C.M.; Frelinger, J.A.; Meyer, A.A. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J. Trauma Acute Care Surg. 2006, 61, 293–298; discussion 298–299. [Google Scholar] [CrossRef]
- Dunn, J.L.; Hunter, R.A.; Gast, K.; Maile, R.; Cairns, B.A.; Schoenfisch, M.H. Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns 2016, 42, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.L.M.; Kartchner, L.B.; Gast, K.; Sessions, M.; Hunter, R.A.; Thurlow, L.; Richardson, A.; Schoenfisch, M.; Cairns, B.A.; Maile, R. Mammalian target of rapamycin regulates a hyperresponsive state in pulmonary neutrophils late after burn injury. J. Leukoc. Biol. 2018, 103, 909–918. [Google Scholar] [CrossRef]
- Dunn, J.L.M.; Kartchner, L.B.; Stepp, W.H.; Glenn, L.I.; Malfitano, M.M.; Jones, S.W.; Doerschuk, C.M.; Maile, R.; Cairns, B.A. Blocking CXCL1-dependent neutrophil recruitment prevents immune damage and reduces pulmonary bacterial infection after inhalation injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L822–L834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.W.; Zhou, H.; Ortiz-Pujols, S.M.; Maile, R.; Herbst, M.; Joyner, B.L., Jr.; Zhang, H.; Kesic, M.; Jaspers, I.; Short, K.A.; et al. Bronchoscopy-derived correlates of lung injury following inhalational injuries: A prospective observational study. PLoS ONE 2013, 8, e64250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neely, C.J.; Kartchner, L.B.; Mendoza, A.E.; Linz, B.M.; Frelinger, J.A.; Wolfgang, M.C.; Maile, R.; Cairns, B.A. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization. PLoS ONE 2014, 9, e85623. [Google Scholar]
- van Duin, D.; Strassle, P.D.; DiBiase, L.M.; Lachiewicz, A.M.; Rutala, W.A.; Eitas, T.; Maile, R.; Kanamori, H.; Weber, D.J.; Cairns, B.A.; et al. Timeline of health care-associated infections and pathogens after burn injuries. Am. J. Infect. Control 2016, 44, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Durbin, E.A.; Gregory, M.S.; Messingham, K.A.; Fontanilla, C.V.; Duffner, L.A.; Kovacs, E.J. The role of interleukin 6 in interferon-gamma production in thermally injured mice. Cytokine 2000, 12, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Segura-Lepe, M.P.; Keun, H.C.; Ebbels, T.M.D. Predictive modelling using pathway scores: Robustness and significance of pathway collections. BMC Bioinform. 2019, 20, 543. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflammation 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, F.; Zou, J.; Coleman, L.G., Jr. Extracellular microvesicles promote microglia-mediated proinflammatory responses to ethanol. J. Neurosci. Res. 2021, 99, 1940–1956. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, X.; Xie, K.P. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol. Cancer 2021, 20, 34. [Google Scholar] [CrossRef]
- Brusselaers, N.; Logie, D.; Vogelaers, D.; Monstrey, S.; Blot, S. Burns, inhalation injury and ventilator-associated pneumonia: Value of routine surveillance cultures. Burns 2012, 38, 364–370. [Google Scholar] [CrossRef]
- Chen, M.C.; Chen, M.H.; Wen, B.S.; Lee, M.H.; Ma, H. The impact of inhalation injury in patients with small and moderate burns. Burns 2014, 40, 1481–1486. [Google Scholar] [CrossRef]
- Cioffi, W.G. What’s new in burns and metabolism. J. Am. Coll. Surg. 2001, 192, 241–254. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruitt, B.A., Jr.; Wolf, S.E. An historical perspective on advances in burn care over the past 100 years. Clin. Plast. Surg. 2009, 36, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Strassle, P.D.; Williams, F.N.; Napravnik, S.; van Duin, D.; Weber, D.J.; Charles, A.; Cairns, B.A.; Jones, S.W. Improved Survival of Patients With Extensive Burns: Trends in Patient Characteristics and Mortality Among Burn Patients in a Tertiary Care Burn Facility, 2004–2013. J. Burn Care Res. 2017, 38, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thombs, B.D.; Singh, V.A.; Halonen, J.; Diallo, A.; Milner, S.M. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: Evidence from a national sample of 31,338 adult patients. Ann. Surg. 2007, 245, 629–634. [Google Scholar] [CrossRef]
- Veeravagu, A.; Yoon, B.C.; Jiang, B.; Carvalho, C.M.; Rincon, F.; Maltenfort, M.; Jallo, J.; Ratliff, J.K. National trends in burn and inhalation injury in burn patients: Results of analysis of the nationwide inpatient sample database. J. Burn Care Res. 2015, 36, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Sun, L.; Guo, F.; Wang, F.; Liu, S.; Liang, X.; Wang, R.S.; Wang, Y.J.; Sun, Y.X. High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PLoS ONE 2012, 7, e50668. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.E.; Krzyzaniak, M.; Costantini, T.W.; De Maio, A.; Baird, A.; Eliceiri, B.P.; Coimbra, R. Vagal nerve stimulation blocks peritoneal macrophage inflammatory responsiveness after severe burn injury. Shock 2012, 38, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnerty, C.C.; Jeschke, M.G.; Herndon, D.N.; Gamelli, R.; Gibran, N.; Klein, M.; Silver, G.; Arnoldo, B.; Remick, D.; Tompkins, R.G.; et al. Temporal cytokine profiles in severely burned patients: A comparison of adults and children. Mol. Med. 2008, 14, 553–560. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Song, J.; Herndon, D.N.; Finnerty, C.C.; Boehning, D.; Barral, J.M.; Jeschke, M.G. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 2008, 30, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasu, E.; Demmelmaier, J.; Kellermann, S.; Holzmann, K.; Kohl, J.; Schmidt, C.Q.; Kalbitz, M.; Gebhard, F.; Huber-Lang, M.S.; Halbgebauer, R. Complement C5a Induces Pro-inflammatory Microvesicle Shedding in Severely Injured Patients. Front. Immunol. 2020, 11, 1789. [Google Scholar] [CrossRef] [PubMed]
- Volanakis, J.E. Complement activation by C-reactive protein complexes. Ann. N. Y. Acad. Sci. 1982, 389, 235–250. [Google Scholar] [CrossRef]
- Thompson, D.; Pepys, M.B.; Wood, S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 1999, 7, 169–177. [Google Scholar] [CrossRef]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Leow, K.Y.; Goh, W.W.; Heng, C.K. Effect of serum amyloid A1 treatment on global gene expression in THP-1-derived macrophages. Inflamm. Res. 2012, 61, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Farre-Alins, V.; Palomino-Antolin, A.; Narros-Fernandez, P.; Lopez-Rodriguez, A.B.; Decouty-Perez, C.; Munoz-Montero, A.; Zamorano-Fernandez, J.; Mansilla-Fernandez, B.; Giner-Garcia, J.; Garcia-Feijoo, P.; et al. Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients. Biomedicines 2021, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Oh, J.H.; Shin, C.Y.; Yoon, H.S.; Lee, D.H.; Chung, J.H. Serum amyloid A1 is induced by UV irradiation and detected by toll-like receptor 4 to causes skin inflammation. J. Dermatol. Sci. 2016, 84, 107–110. [Google Scholar] [CrossRef]
- Fendl, B.; Weiss, R.; Eichhorn, T.; Linsberger, I.; Afonyushkin, T.; Puhm, F.; Binder, C.J.; Fischer, M.B.; Weber, V. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci Rep. 2021, 11, 6996. [Google Scholar] [CrossRef]
- Kulkarni, M.M. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr. Protoc. Mol. Biol. 2011, 94, 25B.10.1–25B.10.17. [Google Scholar] [CrossRef]
- O’Banion, C.P.; Priestman, M.A.; Hughes, R.M.; Herring, L.E.; Capuzzi, S.J.; Lawrence, D.S. Design and Profiling of a Subcellular Targeted Optogenetic cAMP-Dependent Protein Kinase. Cell Chem. Biol. 2018, 25, 100–109.e8. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.; Kaltenbrun, E.; Greco, T.M.; Temple, B.; Herring, L.E.; Cristea, I.M.; Conlon, F.L. Formation of a TBX20-CASZ1 protein complex is protective against dilated cardiomyopathy and critical for cardiac homeostasis. PLoS Genet. 2017, 13, e1007011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Coakley, R.C.; Mascenik, T.; Rowell, T.R.; Davis, E.S.; Rogers, K.; Webster, M.J.; Dang, H.; Herring, L.E.; Sassano, M.F.; et al. Chronic E-cigarette Exposure Alters the Human Bronchial Epithelial Proteome. Am. J. Respir. Crit. Care Med. 2018, 198, 67–76. [Google Scholar] [CrossRef]
- Yi, J.J.; Paranjape, S.R.; Walker, M.P.; Choudhury, R.; Wolter, J.M.; Fragola, G.; Emanuele, M.J.; Major, M.B.; Zylka, M.J. The autism-linked UBE3A T485A mutant E3 ubiquitin ligase activates the Wnt/beta-catenin pathway by inhibiting the proteasome. J. Biol. Chem. 2017, 292, 12503–12515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]



| Gene Name | LFQ Ratio | p-Value |
|---|---|---|
| Haptoglobin (Hp) | 12.0 | 0.003 |
| Serum amyloid A-1 protein (Saa1) | 9.7 | 0.001 |
| Myosin-4 (Myh4) | 7.0 | 0.005 |
| Myosin light chain 1/3 (Myl1) | 6.3 | 0.023 |
| Glycogen phosphorylase (Pygm) | 5.6 | 0.000 |
| Alpha-1-acid glycoprotein 2(Orm2) | 5.0 | 0.014 |
| Serum amyloid A-2 protein (Saa2) | 4.8 | 0.039 |
| Lysophospholipid acyltransferase 5 (Lpcat3) | 3.5 | 0.047 |
| Serum amyloid P-component (Apcs) | 3.0 | 0.000 |
| Complement C1s-B subcomponent (C1sb) | 3.0 | 0.003 |
| Serine protease inhibitor A3N (Serpina3n) | 2.5 | 0.012 |
| Alpha-1-acid glycoprotein 1 (Orm1) | 2.1 | 0.007 |
| Inter-alpha-trypsin inhibitor heavy chain H3 (Itih3) | 2.0 | 0.011 |
| C4b-binding protein (C4bpa) | 1.8 | 0.022 |
| Pyruvate kinase (Pkm) | 1.1 | 0.018 |
| Integrin alpha-IIb (Itga2b) | −1.0 | 0.032 |
| Platelet glycoprotein Ib beta chain (Gp1bb) | −1.1 | 0.009 |
| Gelsolin (Gsn) | −1.1 | 0.011 |
| Integrin-linked protein kinase (Ilk) | −1.2 | 0.023 |
| Cell division control protein 42 homolog (Cdc42) | −1.2 | 0.030 |
| Regulator of G-protein signaling 10 (Rgs10) | −1.2 | 0.037 |
| Beta-parvin (Parvb) | −1.2 | 0.035 |
| Platelet glycoprotein Ib alpha chain (Gp1ba) | −1.2 | 0.044 |
| Lymphocyte antigen 6C1 (Ly6c1) | −1.2 | 0.041 |
| Chloride intracellular channel protein 1 (Clic1) | −1.3 | 0.048 |
| T-complex protein 1 subunit beta (Cct2) | −1.3 | 0.049 |
| Integrin beta-1 (Itgb1) | −1.3 | 0.028 |
| Barrier-to-autointegration factor (Banf1) | −1.4 | 0.008 |
| MOUSE 14–3-3 protein (Ywhah) | −1.5 | 0.011 |
| Translocon-associated protein subunit delta (Ssr4) | −1.6 | 0.027 |
| Syntaxin-11 (Stx11) | −1.7 | 0.045 |
| Cysteine and glycine-rich protein 1 (Csrp1) | −1.7 | 0.043 |
| Calpain small subunit 1 (Capns1) | −1.9 | 0.036 |
| EGF-containing fibulin-like extracellular matrix protein 1 (Efemp1) | −1.9 | 0.016 |
| Phospholipid transfer protein (Pltp) | −2.0 | 0.009 |
| Ras-related protein (Rab21) | −2.0 | 0.039 |
| Leukemia inhibitory factor receptor (Lifr) | −2.3 | 0.011 |
| T-complex protein 1 subunit gamma (Cct3) | −2.4 | 0.037 |
| Retinol dehydrogenase 11 (Rdh11) | −2.5 | 0.021 |
| C-type lectin domain family 11 member A (Clec11a) | −2.6 | 0.035 |
| Transmembrane protein 43 (Tmem43) | −2.6 | 0.021 |
| Choline transporter-like protein 1 (Slc44a1) | −2.8 | 0.044 |
| Ig kappa chain V-V region (1 SV) | −2.9 | 0.025 |
| Calpain-2 catalytic subunit (Capn2) | −3.1 | 0.049 |
| Synaptotagmin-like protein 4 (Sytl4) | −3.1 | 0.014 |
| Protein kinase C alpha type (Prkca) | −3.4 | 0.028 |
| Cytoplasmic FMR1-interacting protein 1 (Cyfip1) | −3.5 | 0.030 |
| Tripeptidyl-peptidase 2 (Tpp2) | −3.7 | 0.002 |
| Integrin alpha-L (Itgal) | −3.8 | 0.030 |
| Vomeromodulin (Bpifb9a) | −3.8 | 0.005 |
| Signal peptidase complex subunit 2 (Spcs2) | −3.9 | 0.006 |
| Dimethylaniline monooxygenase (Fmo5) | −4.4 | 0.000 |
| Sodium/potassium-transporting ATPase subunit alpha-2 (Atp1a2) | −4.4 | 0.007 |
| Gene Name | LFQ Ratio | p-Value |
|---|---|---|
| C-reactive Protein (CRP) | 7.2 | 0.0030 |
| Serum amyloid A-1 (SAA1) | 5.9 | 0.0349 |
| Lactotransferrin (LTF) | 5.8 | 0.0212 |
| HLA class I histocompatibility antigen (HLA-B) | 5.3 | 0.0078 |
| Immunoglobulin lambda constant 6 (IGLC6) | 5.3 | 0.0488 |
| CD59 glycoprotein (CD59) | 4.9 | 0.0388 |
| von Willebrand factor (VWF) | 4.8 | 0.0306 |
| C5a anaphylatoxin chemotactic receptor 1 (C5AR1) | 4.8 | 0.0194 |
| Fatty acid-binding protein, epidermal (FABP5) | 4.3 | 0.0093 |
| Proteoglycan 4 (PRG4) | 2.8 | 0.0387 |
| Monocyte differentiation antigen CD14 (CD14) | 2.7 | 0.0160 |
| Alpha-enolase (ENO1) | 2.4 | 0.0494 |
| Reelin (RELN) | 2.2 | 0.0211 |
| Coagulation factor V (F5) | 2.2 | 0.0075 |
| Fibulin-1 (FBLN1) | 1.7 | 0.0410 |
| CD44 antigen (CD44) | 1.7 | 0.0201 |
| Alpha-2-macroglobulin (A2M) | −1.0 | 0.0246 |
| Alpha-2-HS-glycoprotein (AHSG) | −1.1 | 0.0000 |
| Immunoglobulin lambda-like polypeptide (IGLL5) | −1.5 | 0.0300 |
| Immunoglobulin heavy constant gamma (IGHG3) | −1.7 | 0.0091 |
| Fetuin-B (FETUB) | −2.6 | 0.0340 |
| Actin, alpha skeletal muscle (ACTA1) | −3.2 | 0.0348 |
| Immunoglobulin heavy variable 3–72 OS (IGHV3-72) | −3.3 | 0.0355 |
| %TBSA Injury | Age | Sex # (%) | LOS | Mortality # (%) |
|---|---|---|---|---|
| 18.4 ± 1.7 | 50.3 ±2.2 | M—34 (68) F—16 (32) | 49.4 ± 5.7 | Overall—4 (8) M—2 (5.8) F—2 (12.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maile, R.; Willis, M.L.; Herring, L.E.; Prevatte, A.; Mahung, C.; Cairns, B.; Wallet, S.; Coleman, L.G., Jr. Burn Injury Induces Proinflammatory Plasma Extracellular Vesicles That Associate with Length of Hospital Stay in Women: CRP and SAA1 as Potential Prognostic Indicators. Int. J. Mol. Sci. 2021, 22, 10083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810083
Maile R, Willis ML, Herring LE, Prevatte A, Mahung C, Cairns B, Wallet S, Coleman LG Jr. Burn Injury Induces Proinflammatory Plasma Extracellular Vesicles That Associate with Length of Hospital Stay in Women: CRP and SAA1 as Potential Prognostic Indicators. International Journal of Molecular Sciences. 2021; 22(18):10083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810083
Chicago/Turabian StyleMaile, Robert, Micah L. Willis, Laura E. Herring, Alex Prevatte, Cressida Mahung, Bruce Cairns, Shannon Wallet, and Leon G. Coleman, Jr. 2021. "Burn Injury Induces Proinflammatory Plasma Extracellular Vesicles That Associate with Length of Hospital Stay in Women: CRP and SAA1 as Potential Prognostic Indicators" International Journal of Molecular Sciences 22, no. 18: 10083. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms221810083






