4.1. Maintenance of the Preload According to the Type of Connection
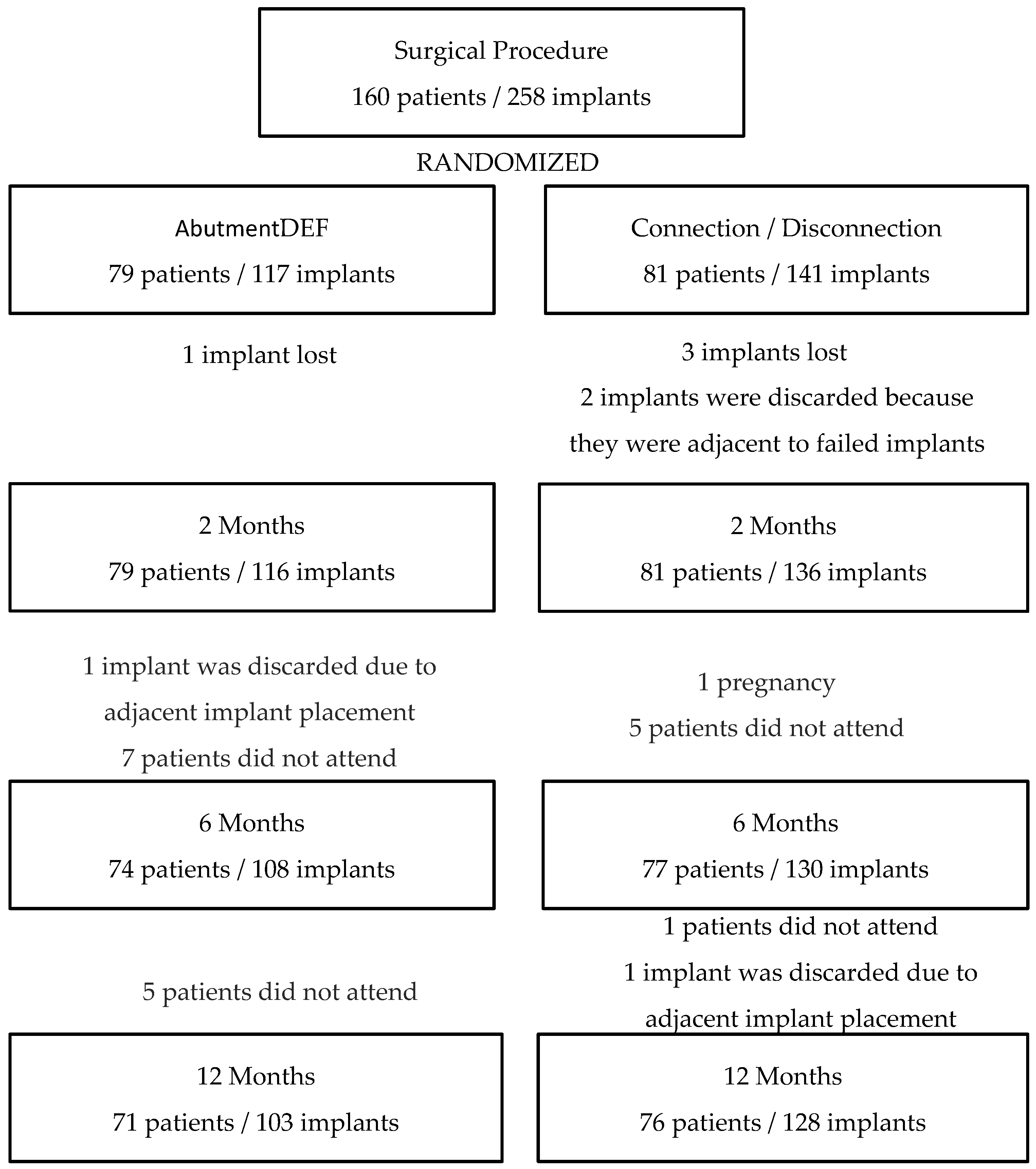
In the present study, peri-implant bone loss was investigated by studying the implants previously described, which were placed in the healed bone and screwed prosthetic at 16 weeks. The patients were randomized after implant placement and after primary stability was measured to avoid bias on the part of the operators at the time of randomization. After this, a homogeneous population was observed with no differences between the groups in regard to age, gender, bone quality, smoking, implant stability, or abutment height (
Table 1). No statistically significant differences were found between the placement of the definitive abutment on the day of surgery without being removed at any other time or following the concept “One Abutment—One Time”, compared to the conventional prosthetic procedure in which the healing screw is removed at least three times. The evolution of the peri-implant bone level is similar in both groups, presenting the greatest loss in the first 6 months, with bone loss being stable in the two groups between 6 and 12 months (
Figure 1). Even without statistically significant differences, the tracking in peri-implant bone loss accumulated at the one-year follow-up in the DEF group (0.36 ± 0.79 mm) is more favorable than the one in the HEA group (0.48 ± 0.71 mm).
Statistical significance was not found between HEA and DEF groups regarding plaque control and bleeding at 6 and 12 months, therefore, the improved response by the DEF group is not due to the better or worse control of plaque by the patient.
When evaluating previous studies with similar characteristics (RCTs), we found a diversity of criteria such as the placement of immediate implants (Canullo [
4]; Degidi [
16]; Grandi [
15]) or healed ridge (Grandi [
13]; Koutouzis [
14]; Molina [
18]), or studies that placed both immediate implants and healed ridge (Luongo [
17]), where we observed no difference between groups during the first 4 months—e.g., the period in which the connection—disconnection process of the healing screw was carried out—however, in the HEA group, there was a greater accumulated bone loss at the three-year mark (0.50 mm) compared to the DEF group (0.07), finding statistical significance (
p = 0.007) [
33]. We found no statistically significant differences between immediate implants associated with immediate loading without occlusion and those placed in a healed ridge, considering both options as treatment alternatives.
Focusing on studies where healed ridge implants were placed [
13,
14,
18,
19], in two of them, Koutouzis [
14] and Praça [
19] statistical significance (
p > 0.05) were not found in peri-implant bone loss. First [
14], they compared the HEA group (0.28 ± 0.16 mm) and the DEF group (0.13 ± 0.20 mm) at the 6 month follow-up (Praça [
19]). At two years of evolution, they found no statistically significant differences (HEA: 0.81 ± 0.15 mm; DEF: 0.61 ± 0.10 mm). In addition, Praça [
19] only found statistical significance between different moments of the study such as between 0 and 2 months (HEA: 0.36 ± 0.10 mm; DEF: 0.70 ± 0.12 mm) and between 2 and 6 months (HEA: 0.65 ± 0.14 mm; DEF: 0.11 ± 0.11 mm); however, in the other two studies, they did find statistical significance: Grandi [
13] (
p < 0.001) found a bone loss of 0.09 ± 0.03 mm in the DEF group and 0.44 ± 0.03 mm in the HEA group, whereas the Molina study [
18](
p = 0.028) found a bone loss of 0.59 ± 0.32 mm in the DEF group and 1.21 ± 0.82 mm in the HEA group. Furthermore, the HEA group presented greater bone loss than the DEF group in both studies. In our study, no statistical differences were found, just as in the aforementioned studies; however, bone loss was greater in the group in which the healing screw was removed and replaced up to three times, unlike in previous studies. In these studies, implants were placed at the crestal level, whereas in our study, implants were placed at the subcrestal level.
A significant factor to take into account is the position of the implant platform at the time of implant placement. In our case, the indication was to place the implant subcrestally due to the expected remodeling of the ridge [
34,
35], with the HEA group at 0.70 mm and the DEF group at 0.79 mm distance between the implant platform and the bone crest on the day of surgery. Clinically, they try to leave the implants 1 mm subcrestal, but the reality is that there are small variations between patients. Even more so considering that it is a multicenter study in which several surgeons participate. It is intended to reflect the reality of daily practice, which is not a mathematical science. On this value, there is also variability between the studies, in which implants were placed in the healed ridge (Grandi [
13]; Koutouzis [
14]; Molina [
18]; Praça [
19]) in all of them, the platform was left at the crestal level; however, in implants that were immediately placed (Canullo [
7]; Degidi [
16]; Grandi [
15], Luongo [
17]), the position of the platform was subcrestal. All studies were performed with internal connection implants and the presence of platform switching.
Another cofactor that can affect bone remodeling and consequently the results of the study is the time of the prosthetic loading, both study groups underwent the prosthetic procedure at the same time, the only difference was that, during this period, in the HEA group the healing screw was removed to perform the impression taking (12 weeks) and subsequent tests until the placement of the definitive prosthesis (16 weeks). In this sense, all studies [
12,
13,
14,
18,
19] performed the prosthetic protocol or placement-disconnection of the healing abutment at the same time.
In our study, as in previous studies [
25,
26,
27], statistical significance (
p = 0.002) has been found when comparing peri-implant bone loss in relation to the height of the intermediate abutments. Galindo [
27], with a follow-up of 18 months, observed better results with regard to peri-implant bone loss in those implants that were placed with abutments of more than 2 mm in height compared to abutments of less than 2 mm (<2 mm: 0.049 ± 0.004; ≥2 mm: 0.024 ± 0.005) (
p = 0.009). White [
25] compared abutments of 1 vs. 3 mm, finding statistical significance both at 3 months (0.83 ± 0.19 mm vs. 0.14 ± 0.08 mm) and at 6 months of follow-up (0.91 ± 0.19 mm vs. 0.11 ± 0.09 mm). Novoa [
26], after a 3-year follow-up comparing abutments of 1 vs. 2.5 mm in height, concluded that peri-implant bone loss is greater in those cases in which short abutments (<2 mm) are used, finding statistical significance at 12 (1 mm: 0.82 ± 0.10 mm; 2.5 mm: 0.2 ± 0.28 mm), 24 (1 mm: 1.27 ± 1.02 mm; 2.5 mm: 0.22 ± 0.37 mm) and 36 (1 mm: 1.23 ± 1.61 mm; 2.5 mm: 0.35 ± 0.62 mm) months of follow-up.
Due to the fact that we have not found differences between placing the abutment on the day of surgery or later, the selection of the abutment and its height can be deferred to the moment when the peri-implant tissues are completely healed after surgery; independently of that we use a conventional workflow or CAD/CAM techniques for prosthetic rehabilitation [
36].
The use of resonance frequency analysis (RFA) is the most reliable method to assess implant stability over time [
37]. In our study, the values were recorded on the day of surgery, at 6 months, and at 12 months post-implant placement. On the day of surgery, all records (RFA) were taken with Smart-Pegs directly from the implant, whereas at the 6 and 12-month revisions, the Smart-Pegs were screwed to the prosthesis abutments. The values at 2 months of follow-up were not recorded since, at that time, it would have been necessary to measure the implant in the HEA group and the abutment in the DEF group since it was never removed, thus avoiding the existing differences in the values measured to implant or abutment [
38]. The values were considered high on the day of surgery (HEA: 77 ± 9.30 ISQ; DEF: 78.36 ± 8.13 ISQ) being slightly higher in the DEF group, these values remained stable both in the 6 month review (HEA: 79.67 ± 8.18 ISQ; DEF: 76.54 ± 9.41 ISQ) and in the 12 month review (HEA: 80.07 ± 9.01 ISQ; DEF: 76.51 ± 12.46 ISQ). A slight decrease was observed in the DEF group after 6 months, a common trend after osseointegration when high primary stability values are obtained [
39]. statistical significance (
p = 0.731) was not found between groups (HEA/DEF) or between the different times of the study (surgery, 6 months, and 12 months) in each group. The results of our study are similar to those presented by Praça [
19], a study with similar methodology comparing HEA and DEF groups, which also specifically presented similar results both on the day of surgery (HEA: 77.1 ± 5.5 ISQ; DEF: 75.1 ± 5.8 ISQ) and 6 month follow-up (HEA: 72.0 ± 3.2 ISQ; DEF: 74.3 ± 2.8 ISQ) without finding statistical significance concerning the RFA values between the groups, nor between the different moments of the study, presenting little variability between the day of surgery and 6 months.
In Cornelini’s study [
40], whose objective was to evaluate the success and stability of implants placed in posterior mandibular sectors associated with immediate loading at 12 months, results were observed on the day of surgery (72 ± 5.7 ISQ) and their evolution up to 12 months (74.5 ± 7.3 ISQ) concluded with results similar to ours, although they are not comparable due to the methodological differences between both studies. In a previous study of ours [
41], with screw-shaped, tissue level implants (Essential Cone, Klockner Implant System, SOADCO, Engordany, Andorra) with two different collar heights (0.7 mm or 1.5 mm), high ISQ values were also obtained, which we consider to be directly related to the success of dental implant treatments. The ISQ on the day of the surgery was 68.61 ± 10.35; at 8 weeks, it was 70.61 ± 7.5; and at 2 years, it was 74.39 ± 9.64.