Neonatal Respiratory Distress and Airway Emergency: Report of Two Cases
Abstract
:1. Introduction
2. Case Presentation
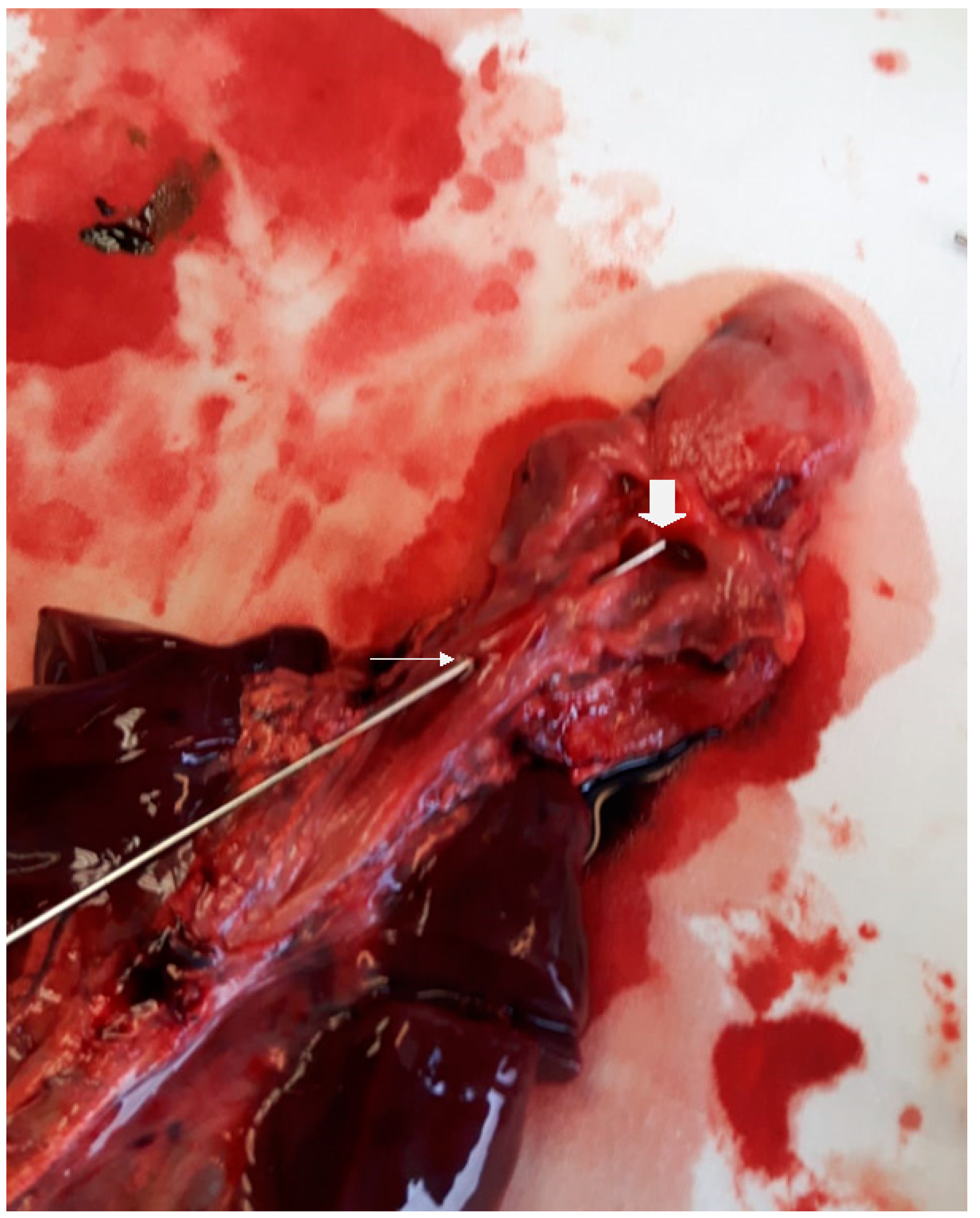
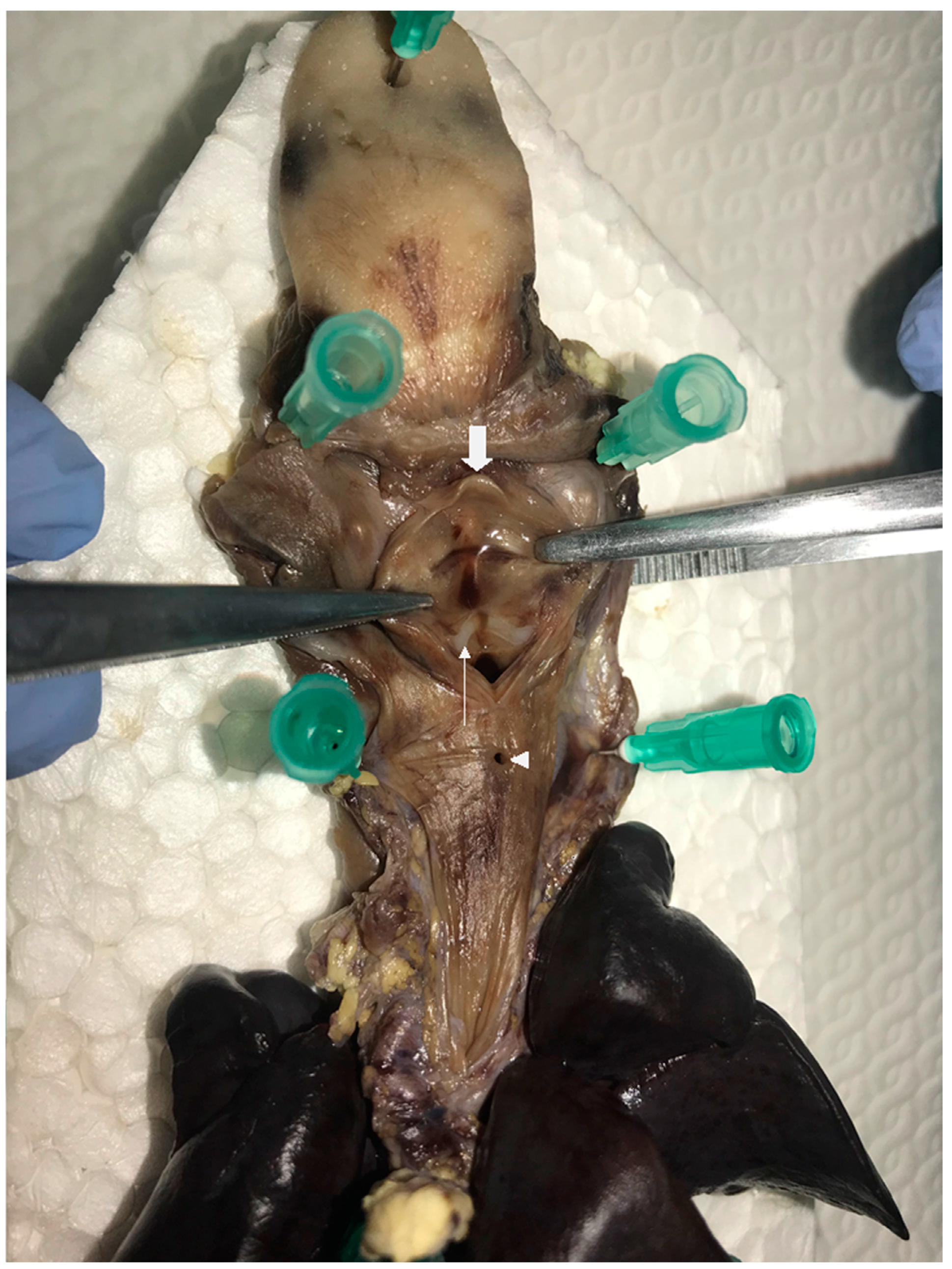
2.1. Case One
2.2. Case Two
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roybal, J.L.; Liechty, K.W.; Hedrick, H.L.; Bebbington, M.W.; Johnson, M.P.; Coleman, B.G.; Adzick, N.S.; Flake, A.W. Predicting the severity of congenital high airway obstruction syndrome. J. Pediatr. Surg. 2010, 45, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Hartnick, C.J.; Rutter, M.; Lang, F.; Willging, J.P.; Cotton, R.T. Congenital high airway obstruction syndrome and airway reconstruction: An evolving paradigm. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 567–570. [Google Scholar] [CrossRef] [Green Version]
- de Groot-van der Mooren, M.D.; Haak, M.C.; Lakeman, P.; Cohen-Overbeek, T.E.; van der Voorn, J.P.; Bretschneider, J.H.; van Elburg, R.M. Tracheal agenesis: Approach towards this severe diagnosis. Case report and review of the literature. Eur. J. Pediatr. 2012, 171, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.; Somme, S.; Crombleholme, T.M. Airway compromise in the fetus and neonate: Prenatal assessment and perinatal management. Semin. Fetal Neonatal Med. 2016, 21, 230–239. [Google Scholar] [CrossRef]
- Ashraf, A.; Abdelrahman, A.M.; Senna, A.; Alsaad, F. Congenital high airway obstruction syndrome (CHAOS): No intervention, no survival—A case report and literature review. Case Rep. Radiol. 2020, 2020, 1036073. [Google Scholar] [CrossRef]
- Payne, W.A. Congenital absence of the trachea. Brooklyn Med. J. 1900, 14, 568. [Google Scholar]
- Floyd, J.; Campbell, D.C., Jr.; Dominy, D.E. Agenesis of the trachea. Am. Rev. Respir. Dis. 1962, 86, 557–560. [Google Scholar] [CrossRef]
- Hill, S.A.; Milam, M.; Manaligod, J.M. Tracheal agenesis: Diagnosis and management. Int. J. Pediatr. Otorhinolaryngol. 2001, 59, 63–68. [Google Scholar] [CrossRef]
- Faro, R.S.; Goodwin, C.D.; Organ, C.H., Jr.; Hall, R.T.; Holder, T.M.; Ashcraft, K.W.; Amoury, R.A. Tracheal agenesis. Ann. Thorac. Surg. 1979, 28, 295–299. [Google Scholar] [CrossRef]
- Smith, M.M.; Huang, A.; Labbé, M.; Lubov, J.; Nguyen, L.H.P. Clinical presentation and airway management of tracheal atresia: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2017, 101, 57–64. [Google Scholar] [CrossRef]
- Fausett, S.R.; Klingensmith, J. Compartmentalization of the foregut tube: Developmental origins of the trachea and esophagus. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.I.; Bain, A.D. Congenital atresia of larynx: A report of nine cases. Ann. Otol. Rhinol. Laryngol. 1965, 74, 338–349. [Google Scholar] [CrossRef]
- Tazuke, Y.; Okuyama, H.; Uehara, S.; Ueno, T.; Nara, K.; Yamanaka, H.; Kawahara, H.; Kubota, A.; Usui, N.; Soh, H.; et al. Long-term outcomes of four patients with tracheal agenesis who underwent airway and esophageal reconstruction. J. Pediatr. Surg. 2015, 50, 2009–2011. [Google Scholar] [CrossRef]
- Mohammed, H.; West, K.; Bewick, J.; Wickstead, M. Tracheal agenesis, a frightening scenario. J. Laryngol. Otol. 2016, 130, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Henick, D.H.; Holinger, L.D. Laryngeal development. In Pediatric Laryngology and Bronchoesophagology; Holinger, L.D., Lusk, R.P., Green, C.G., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 1–17. ISBN 978-039-751-650-6. [Google Scholar]
- Yamamoto, M.; Honkura, Y.; Rodríguez-Vázquez, J.F.; Murakami, G.; Katori, Y.; Cho, B.H.; Abe, S. Switching of the laryngeal cavity from the respiratory diverticulum to the vestibular recess: A study using serial sagittal sections of human embryos and fetuses. J. Voice 2016, 30, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merei, J.M.; Hasthorpe, S.; Farmer, P.; Hutson, J.M. Embryogenesis of tracheal atresia. Anat. Rec. 1998, 252, 271–275. [Google Scholar] [CrossRef]
- Domyan, E.T.; Ferretti, E.; Throckmorton, K.; Mishina, Y.; Nicolis, S.K.; Sun, X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 2011, 138, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Sattler, C.; Chiao, F.; Stein, D.; Murphy, D. Life-Saving Esophageal Intubation in Neonate with Undiagnosed Tracheal Agenesis: A Case Report. A A Case Rep. 2017, 9, 31–34. [Google Scholar] [CrossRef]
- Mudaliyar, U.S.; Sreedhar, S. Chaos syndrome. BJR Case Rep. 2017, 3, 20160046. [Google Scholar] [CrossRef]
- Grass, B.; Simma, L.; Reinehr, M.; Zimmermann, U.; Gysin, C.; Henze, G.; Cannizzaro, V. Two case reports of unexpected tracheal agenesis in the neonate: 3 C’s beyond algorithms for difficult airway management. BMC Pediatr. 2017, 17, 49:1–49:6. [Google Scholar] [CrossRef] [Green Version]
- Nolan, H.R.; Gurria, J.; Peiro, J.L.; Tabbah, S.; Diaz-Primera, R.; Polzin, W.; Habli, M.; Lim, F.Y. Congenital high airway obstruction syndrome (CHAOS): Natural history, prenatal management strategies, and outcomes at a single comprehensive fetal center. J. Pediatr. Surg. 2019, 54, 1153–1158. [Google Scholar] [CrossRef]
- Krishna, S.G.; Bryant, J.F.; Tobias, J.D. Management of the difficult airway in the pediatric patient. J. Pediatr. Intensive Care 2018, 7, 115–125. [Google Scholar] [CrossRef]
- Oppido, G.; Pace Napoleone, C.; Loforte, A.; Baroncini, S.; Lima, M.; Gargiulo, G. Complex double-outlet right ventricle repair in a neonate with complete tracheal agenesis. J. Thorac. Cardiovasc. Surg. 2004, 127, 283–285. [Google Scholar] [CrossRef] [Green Version]
- Willer, B.L.; Bryan, K.G.; Parakininkas, D.E.; Uhing, M.R.; Staudt, S.R.; Dominguez, K.M.; McCormick, M.E.; Mitchell, M.E.; Densmore, J.C.; Oldham, K.T.; et al. Anesthetic management of an infant with postnatally diagnosed tracheal agenesis undergoing tracheal reconstruction: A case report. A A Case Rep. 2017, 9, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Densmore, J.C.; Oldham, K.T.; Dominguez, K.M.; Berdan, E.R.; McCormick, M.E.; Beste, D.J.; Amos, L.B.; Lang, C.A.; Woods, R.K.; Kouretas, P.C.; et al. Neonatal esophageal trachealization and esophagocarinoplasty in the treatment of flow-limited Floyd II tracheal agenesis. J. Thorac. Cardiovasc. Surg. 2017, 153, e121–e125. [Google Scholar] [CrossRef] [Green Version]
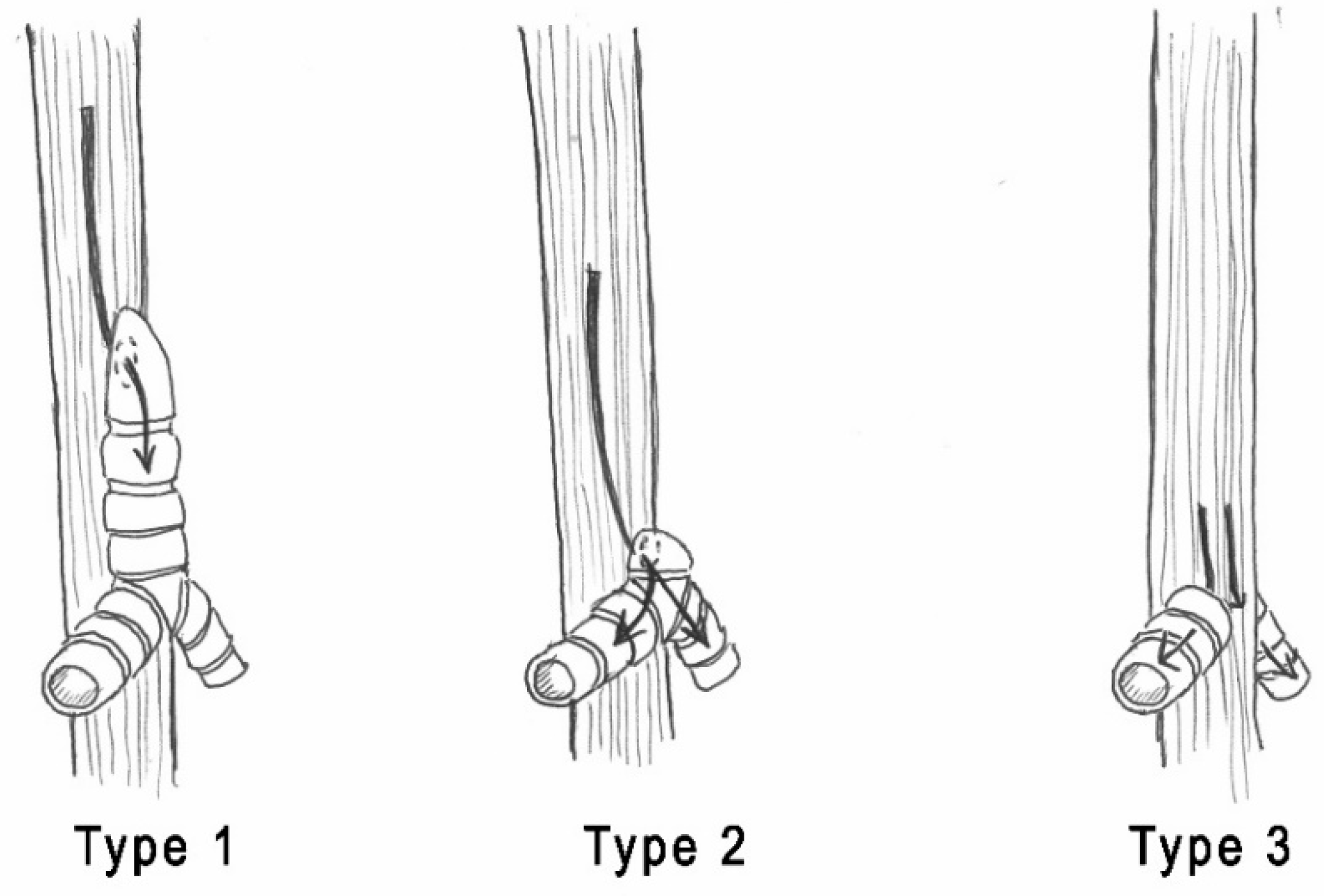
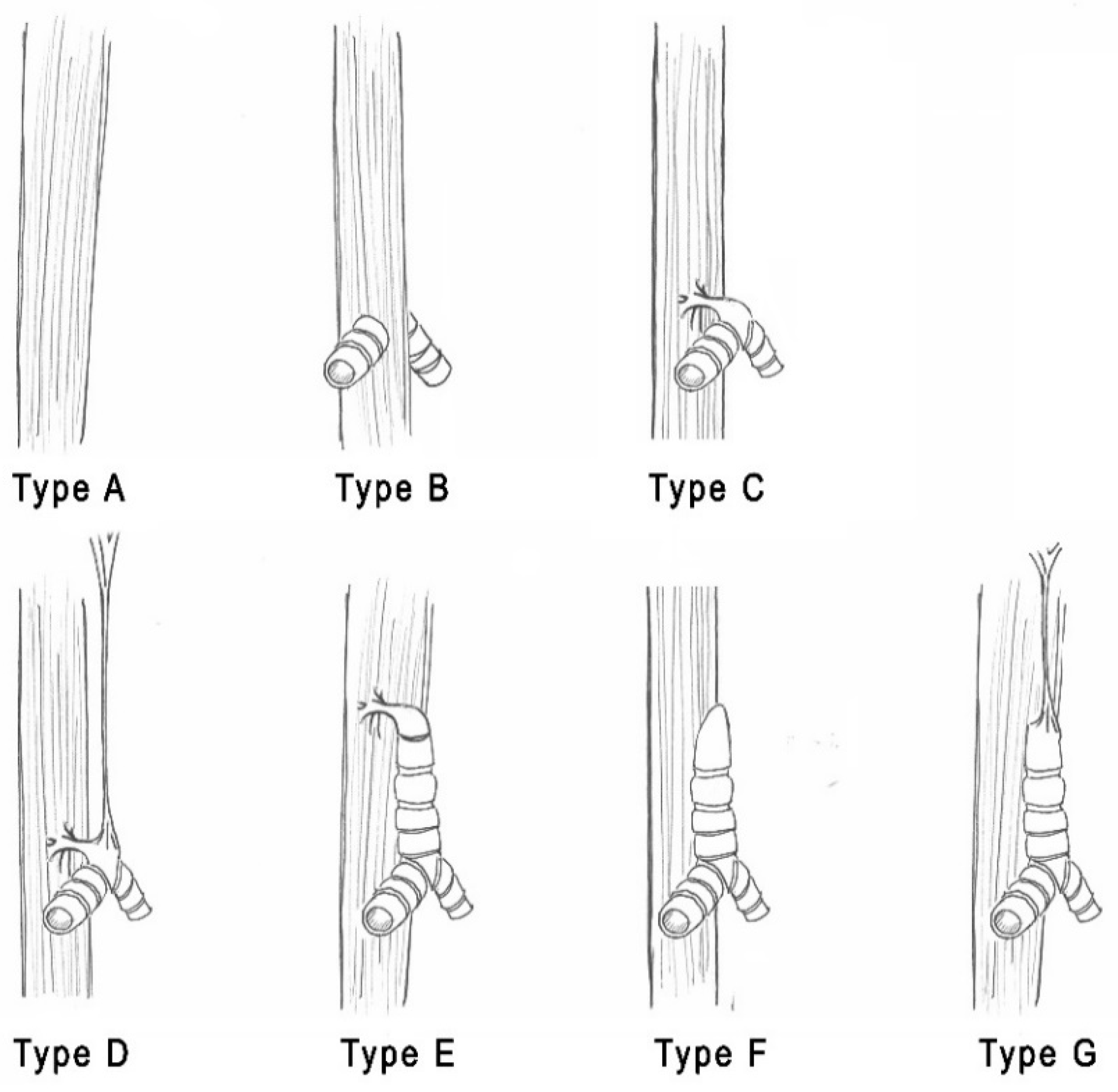

| Smith & Bain [12] | Hartnick et al. [2] |
|---|---|
| Type 1: complete LA with midline fusion of arytenoid cartilages and intrinsic muscles. | Type 1: complete LA without an esophageal fistula. |
| Type 2: subglottic obstruction where the dome-shaped cricoid cartilage obstructs the lumen. | Type 2: complete LA with a TEF. |
| Type 3: occlusion of anterior fibrous membranes and fusion of arytenoid cartilages at the level of the vocal process. | Type 3: near-complete high upper airway obstruction. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresciani, L.; Grazioli, P.; Bosio, R.; Chirico, G.; Zambelloni, C.; Santoro, A.; Baronchelli, C.; Redaelli de Zinis, L.O. Neonatal Respiratory Distress and Airway Emergency: Report of Two Cases. Children 2021, 8, 255. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040255
Bresciani L, Grazioli P, Bosio R, Chirico G, Zambelloni C, Santoro A, Baronchelli C, Redaelli de Zinis LO. Neonatal Respiratory Distress and Airway Emergency: Report of Two Cases. Children. 2021; 8(4):255. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040255
Chicago/Turabian StyleBresciani, Lorenzo, Paola Grazioli, Roberta Bosio, Gaetano Chirico, Cesare Zambelloni, Amerigo Santoro, Carla Baronchelli, and Luca O. Redaelli de Zinis. 2021. "Neonatal Respiratory Distress and Airway Emergency: Report of Two Cases" Children 8, no. 4: 255. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040255







