Advanced Characterization of Organic Matter Decaying during Composting of Industrial Waste Using Spectral Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Analysis
2.2.1. Physical-Chemical Analysis
2.2.2. X-ray Diffraction Analysis
2.2.3. Infrared Spectroscopy Analysis
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physical-Chemical Properties
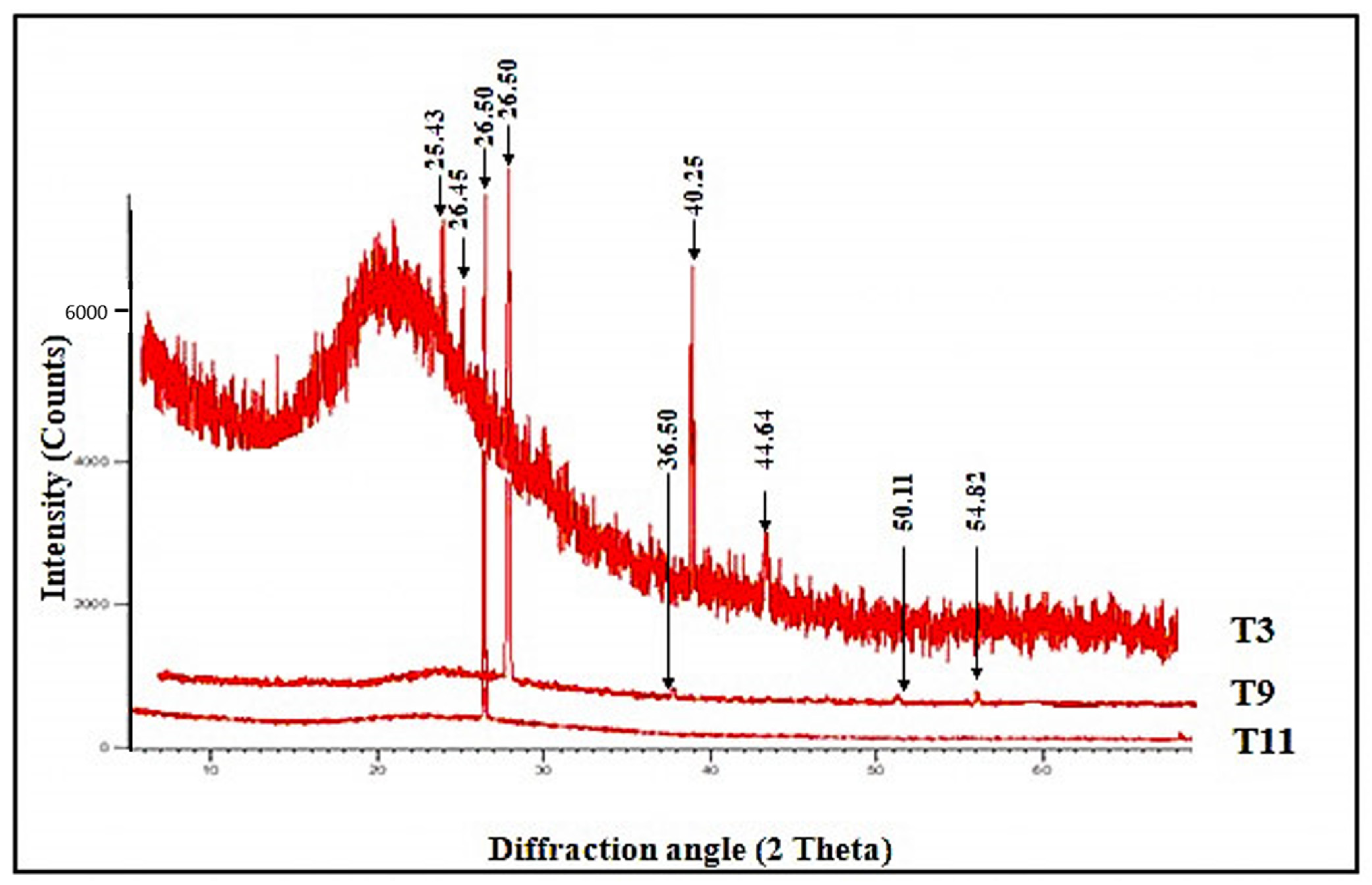
3.2. X-ray Diffraction Analysis
3.3. Infrared Spectroscopy Analysis
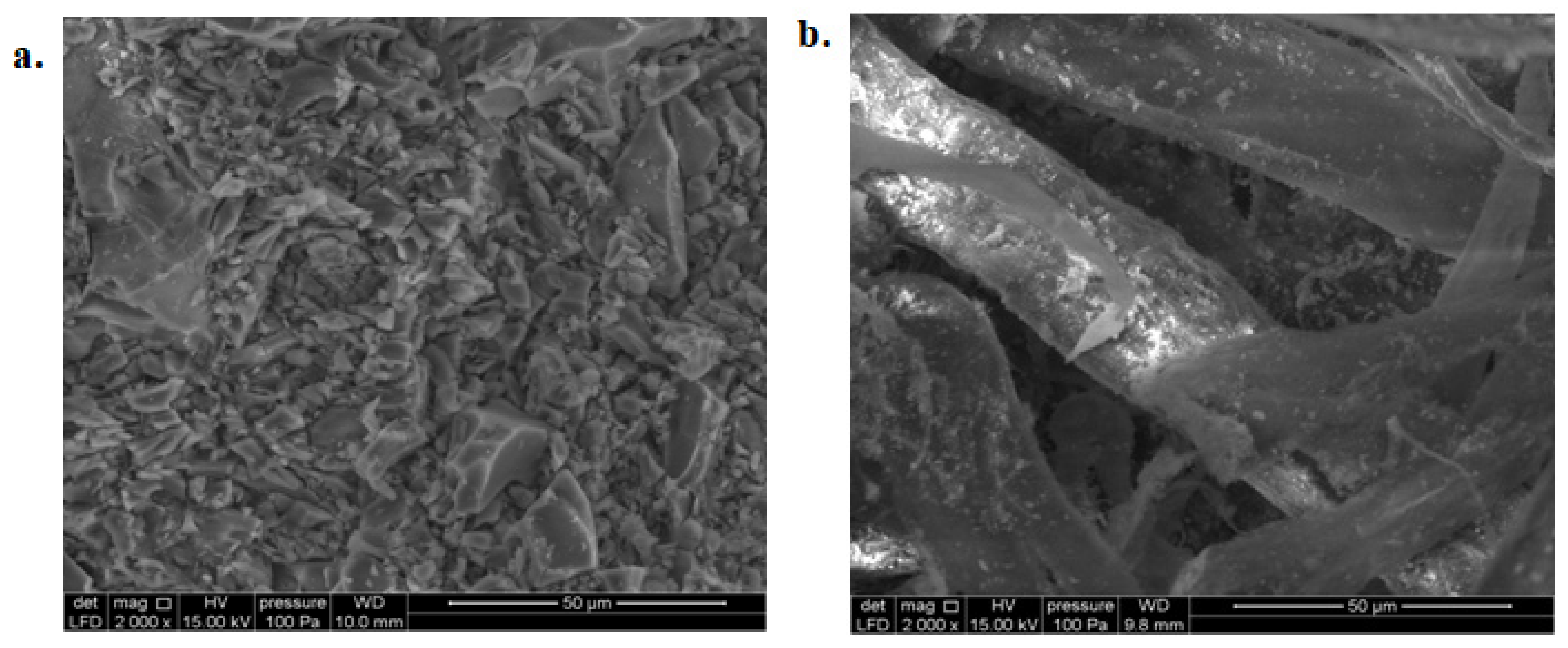
3.4. Scanning Electron Microscopy (SEM)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahra El Ouaqoudi, F.; El Fels, L.; Lemée, L.; Amblès, A.; Hafidi, M. Evaluation of Lignocelullose Compost Stability and Maturity Using Spectroscopic (FTIR) and Thermal (TGA/TDA) Analysis. Ecol. Eng. 2015, 75, 217–222. [Google Scholar] [CrossRef]
- Talouizte, H.; Merzouki, M.; Benlemlih, M. Treatment of Real Textile Wastwater Using SBR Technology: Effect of Sludge Age and Operational Parameters. J. Biotechnol. Lett. 2017, 4, 79–83. [Google Scholar]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil Structure and the Effect of Management Practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Albrecht, R.; Joffre, R.; Gros, R.; Le Petit, J.; Terrom, G.; Périssol, C. Efficiency of Near-Infrared Reflectance Spectroscopy to Assess and Predict the Stage of Transformation of Organic Matter in the Composting Process. Bioresour. Technol. 2008, 99, 448–455. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Y.; Long, Y.; Zhu, Y.; Wang, H.; Lu, W. Evaluation of the Biological Stability of Waste during Landfill Stabilization by Thermogravimetric Analysis and Fourier Transform Infrared Spectroscopy. Bioresour. Technol. 2011, 102, 9403–9408. [Google Scholar] [CrossRef]
- Albrecht, R.; Le Petit, J.; Calvert, V.; Terrom, G.; Périssol, C. Changes in the Level of Alkaline and Acid Phosphatase Activities during Green Wastes and Sewage Sludge Co-Composting. Bioresour. Technol. 2010, 101, 228–233. [Google Scholar] [CrossRef]
- Crecchio, C.; Curci, M.; Pizzigallo, M.D.R.; Ricciuti, P.; Ruggiero, P. Effects of Municipal Solid Waste Compost Amendments on Soil Enzyme Activities and Bacterial Genetic Diversity. Soil Biol. Biochem. 2004, 36, 1595–1605. [Google Scholar] [CrossRef]
- Peláez, C.; Mejía, A.; Planas, A. Development of a Solid Phase Kinetic Assay for Determination of Enzyme Activities during Composting. Process Biochem. 2004, 39, 971–975. [Google Scholar] [CrossRef]
- Vergnoux, A.; Guiliano, M.; Le Dréau, Y.; Kister, J.; Dupuy, N.; Doumenq, P. Monitoring of the Evolution of an Industrial Compost and Prediction of Some Compost Properties by NIR Spectroscopy. Sci. Total Environ. 2009, 407, 2390–2403. [Google Scholar] [CrossRef]
- Aguelmous, A.; Lahsaini, S.; El Fels, L.; Souabi, S.; Zamama, M.; Hafidi, M. Biodegradation Assessment of Biological Oil Sludge from a Petroleum Refinery. J. Mater. Environ. Sci. 2016, 7, 3421–3430. [Google Scholar]
- Temporal-lara, B.; Melendez-pastor, I.; Gómez, I.; Navarro-pedreño, J. Wastewater Biosolid Composting Optimization Based on UV-VNIR Spectroscopy Monitoring. Sensors 2016, 16, 1919. [Google Scholar] [CrossRef] [Green Version]
- Ilani, T.; Herrmann, I.; Karnieli, A.; Arye, G. Characterization of the Biosolids Composting Process by Hyperspectral Analysis. Waste Manag. 2016, 48, 106–114. [Google Scholar] [CrossRef]
- Ueno, M.; Taira, E.; Kawamitsu, Y.; Komiya, Y.; Kikuchi, K. Application of FT-NIR Spectroscopy to the Evaluation of Compost Quality. Eng. Agric. Environ. Food 2008, 1, 51–56. [Google Scholar] [CrossRef]
- Afnor. Amendements du sol et Support de Culture-Préparation des Echantillons Pour les Essais Physiques et Chimiques, Détermination de la Teneur en Matière Sèche, du Taux D’humidité et de la Masse Volumique Compactée en Laboratoire; The French Association for Standardization: Paris, France, 2000. [Google Scholar]
- Biyada, S.; Merzouki, M.; Demcenko, T.; Vasiliauskiene, D.; Urbonavicius, J.; Marciulaitiene, E.; Vasarevicius, S.; Benlemlih, M. Evolution of Microbial Composition and Enzymatic Activities during the Composting of Textile Waste. Appl. Sci. 2020, 10, 3758. [Google Scholar] [CrossRef]
- Albrecht, R.; Le Petit, J.; Terrom, G.; Périssol, C. Comparison between UV Spectroscopy and Nirs to Assess Humification Process during Sewage Sludge and Green Wastes Co-Composting. Bioresour. Technol. 2011, 102, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Boukir, A.; Mehyaoui, I.; Fellak, S.; Asia, L.; Doumenq, P. The Effect of the Natural Degradation Process on the Cellulose Structure of Moroccan Hardwood Fiber: A Survey on Spectroscopy and Structural Properties. Mediterr. J. Chem. 2019, 8, 179–190. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural Characterization of Argania Spinosa Moroccan Wooden Artifacts during Natural Degradation Progress Using Infrared Spectroscopy (ATR-FTIR) and X-ray Diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, W.; Gong, X.; Li, Y.; Wu, C.; Ren, N. Spectral Study of Dissolved Organic Matter in Biosolid during the Composting Process Using Inorganic Bulking Agent: UV e Vis, GPC, FTIR and EEM. Int. Biodeterior. Biodegrad. 2013, 85, 617–623. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Nour El-Din, M.; Refaat, B.M.; Abdel-Shakour, E.H.; Ewais, E.E.D.; Alrefaey, H.M.A. Biotechnological Application of Thermotolerant Cellulose-Decomposing Bacteria in Composting of Rice Straw. Ann. Agric. Sci. 2016, 61, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Hachicha, R.; Hachicha, S.; Trabelsi, I.; Woodward, S.; Mechichi, T. Evolution of the Fatty Fraction during Co-Composting of Olive Oil Industry Wastes with Animal Manure: Maturity Assessment of the End Product. Chemosphere 2009, 75, 1382–1386. [Google Scholar] [CrossRef]
- López-gonzález, J.A.; Vargas-garcía, M.C.; López, M.J.; Suárez-estrella, F.; Jurado, M.; Moreno, J. Biodiversity and Succession of Mycobiota Associated to Agricultural Lignocellulosic Waste-Based Composting. Bioresour. Technol. 2015, 187, 305–313. [Google Scholar] [CrossRef]
- Srivastava, V.; Goel, G.; Kumar, V.; Pratap, R.; De Araujo, A.S.F.; Singh, P. Analysis and Advanced Characterization of Municipal Solid Waste Vermicompost Maturity for a Green Environment. J. Environ. Manag. 2020, 255, 109914. [Google Scholar] [CrossRef]
- Soobhany, N.; Gunasee, S.; Pooja, Y.; Joyram, H.; Raghoo, P. Spectroscopic, Thermogravimetric and Structural Characterization Analyses for Comparing Municipal Solid Waste Composts and Vermicomposts Stability and Maturity. Bioresour. Technol. 2017, 236, 11–19. [Google Scholar] [CrossRef]
- Sharma, A.; Ganguly, R.; Kumar, A. Spectral Characterization and Quality Assessment of Organic Compost for Agricultural Purposes. Int. J. Recycl. Org. Waste Agric. 2019, 8, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.C. Structural Characterization of Fulvic Acids during Composting of Sewage Sludge. Process Biochem. 2005, 40, 1693–1700. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Luis, J.; Luisa, M. Artificial Aging Paper to Assess Long-Term Effects of Conservative Treatment. Monitoring by Infrared Spectroscopy (ATR-FTIR), X-ray Diffraction (XRD), and Energy Dispersive X-ray Fl Uorescence (EDXRF) ☆. Microchem. J. 2016, 124, 646–656. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Lakhiari, H.; Kerbal, A.; Doumenq, P.; Mille, G.; Luisa, M.; Carvalho, D. Conservation of Moroccan Manuscript Papers Aged 150, 200 and 800 Years. Analysis by Infrared Spectroscopy (ATR-FTIR), X-ray Diffraction (XRD), and Scanning Electron Microscopy Energy Dispersive Spectrometry (SEM-EDS). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1038–1046. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y. Determination of Maturity in the Vermicompost Produced from Palm Oil Mill Effluent Using Spectroscopy, Structural Characterization and Thermogravimetric Analysis. Ecol. Eng. 2015, 84, 515–519. [Google Scholar] [CrossRef]
- Garriga, P.; Colom, X.; Carrillo, F.; Nogue, F. Structural Analysis of Photodegraded Wood by Means of FTIR Spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Zghari, B.; Doumenq, P.; Romane, A.; Boukir, A. GC-MS, FTIR and 1 H, 13 C NMR Structural Analysis and Identification of Phenolic Compounds in Olive Mill Wastewater Extracted from Oued Oussefrou Effluent (Beni Mellal-Morocco). J. Mater. Environ. Sci. 2017, 8, 4496–4509. [Google Scholar]
- Hajji, L.; Boukir, A.; Assouik, J.; Kerbal, A.; Kajjout, M. A Multi-Analytical Approach for the Evaluation of the Efficiency of the Conservation—Restoration Treatment of Moroccan Historical Manuscripts Dating to the 16th, 17th, and 18th Centuries. Appl. Spectrosc. 2015, 69, 920–938. [Google Scholar] [CrossRef] [PubMed]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Vibrational Spectroscopy; Springer: Dordrecht, The Netherlands, 2019; ISBN 1057001821. [Google Scholar]
- Acharya, S.; Hu, Y.; Moussa, H.; Abidi, N. Preparation and Characterization of Transparent Cellulose Films Using an Improved Cellulose Dissolution Process. J. Appl. Polym. Sci. 2017, 44871, 1–12. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Application of ATR—FTIR Spectroscopy to the Analysis of Tannins in Historic Leathers: The Case Study of the Upholstery from the 19th Century Portuguese Royal Train. Vib. Spectrosc. 2014, 74, 98–103. [Google Scholar] [CrossRef]
- Droussi, Z.; Valeria, D.; Rosaria, M.; Hafidi, M.; Ouatmane, A. Study of the Biodegradation and Transformation of Olive-Mill Residues during Composting Using FTIR Spectroscopy and Differential Scanning Calorimetry. J. Hazard. Mater. 2009, 164, 1281–1285. [Google Scholar] [CrossRef]
- Katusiimeh, M.W.; Burger, K.; Mol, A.P.J. Informal Waste Collection and Its Co-Existence with the Formal Waste Sector: The Case of Kampala, Uganda. Habitat Int. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Chang, C.; Laird, D.A. Near-Infrared Reflectance Spectroscopic Analysis of Soil c and n. Soil Sci. 2002, 167, 110–116. [Google Scholar] [CrossRef]
- Malley, D.F.; Mcclure, C.; Martin, P.D. Compositional Analysis of Cattle Manure During Composting Using a Field—Portable Near—Infrared Spectrometer. Commun. Soil Sci. Plant Anal. 2005, 36, 455–475. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.C.; Hafidi, M. Chemical and Spectroscopic Analysis of Organic Matter Transformation during Composting of Sewage Sludge and Green Plant Waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]




| Physical-Chemical Parameters | Green Waste | Textile Waste | Paper and Cardboard Waste |
|---|---|---|---|
| Moisture% | 60.56 ± 0.04 | 50.26 ± 0.05 | 10.23 ± 1.06 |
| pH | 6.80 ± 0.40 | 7.30 ± 0.11 | 7.20 ± 0.01 |
| Total Organic Carbon% | 44.56 ± 0.60 | 30.26 ± 0.36 | 40.26 ± 0.04 |
| Total Kjeldahl Nitrogen% | 1.23 ± 1.00 | 0.53 ± 0.80 | 1.01 ± 0.20 |
| C/N Ratio | 36.22 ± 0.01 | 57.09 ± 0.15 | 39.86 ± 0.18 |
| Time (Months) | TOC (%) | TN (%) | C/N Ratio | NH4+/NO3− | Ash (%) | Moisture (%) | Temperature (°C) | pH | Cellulose Activity (U g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Initial | 32.64 ± 0.96 | 0.56 ± 0.04 | 58.29 | 13.79 | 41.25 | 69.90 ± 3.00 | 10.50 ± 2.10 | 8.37 ± 0.10 | 0.41 |
| 3 | 28.26 ± 0.50 | 0.76 ± 0.01 | 37.18 | 6.09 | 49.13 | 34.55 ± 3.80 | 25.00 ± 2.00 | 7.53 ± 0.50 | 30.55 |
| 9 | 23.91 ± 0.45 | 1.17 ± 0.04 | 20.44 | 0.89 | 56.96 | 45.91 ± 2.30 | 42.00 ± 2.90 | 6.86 ± 0.10 | 42.59 |
| 11 | 23.53 ± 0.12 | 1.10 ± 0.02 | 21.39 | 0.28 | 57.65 | 37.24 ± 3.10 | 29.00 ± 1.20 | 6.02 ± 0.23 | 5.84 |
| Time (Months) | Abs472nm | Abs664nm | Q4/Q6 |
|---|---|---|---|
| Initial | 0.20 | 0.003 | 66.00 |
| 3 | 0.44 | 0.030 | 14.67 |
| 9 | 0.70 | 0.080 | 8.75 |
| 11 | 0.80 | 0.090 | 8.89 |
| Wavenumber (cm−1) | Band Assignements |
|---|---|
| 3700–3000 | ѵ(OH) hydroxyl groups in lignin, cellulose and hemicelluloses Intermolecular hydrogen-bonded |
| 1700–1600 | ѵC=O ester in acetoxy groups (H3C–(C=O) –O–) in hemicellulose, ѵC=O in quinone or p-quinone |
| 1400–1300 | δC–H and δsCH3 in cellulose and hemicelluloses |
| 1120 | CH stretching vibrations in different groups of lignin and cellulose and hemicelluloses |
| 1030 | C–O–C stretching vibration of lignin and polysaccharides |
| 787 | CH2 rocking vibration in cellulose (cellulose Iβ appear generally as a tiny peak at 750 cm−1 and 3240 cm−1) |
| Mean | St. Dev. | CV (%) | ANOVA (p) | R2 | RMSEP * | |
|---|---|---|---|---|---|---|
| Time | 5.75 | 1.90 | 33.04 | <0.05 | 0.90 | 1.35 |
| TOC | 27.08 | 0.62 | 2.29 | <0.05 | 0.98 | 0.58 |
| TN | 0.89 | 0.07 | 7.87 | 0.004 | 0.98 | 0.07 |
| C/N | 34.32 | 3.28 | 0.82 | 0.002 | 0.98 | 3.10 |
| NH4+/NO3− | 5.26 | 0.67 | 12.74 | <0.05 | 0.97 | 0.64 |
| T° | 23.62 | 4.12 | 17.44 | 0.045 | 0.77 | 3.89 |
| Ph | 7.19 | 0.40 | 5.56 | <0.05 | 0.68 | 0.38 |
| Moisture | 46.90 | 8.33 | 17.76 | 0.143 ns | 0.37 | 7.88 |
| Q4/Q6 | 24.23 | 5.31 | 21.91 | 0.021 | 0.79 | 5.02 |
| Cellulase activity | 19.85 | 9.23 | 46.49 | <0.05 | 0.98 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biyada, S.; Merzouki, M.; Imtara, H.; Alajmi, M.F.; Elkarrach, K.; Mechchate, H.; Conte, R.; Benlemlih, M. Advanced Characterization of Organic Matter Decaying during Composting of Industrial Waste Using Spectral Methods. Processes 2021, 9, 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9081364
Biyada S, Merzouki M, Imtara H, Alajmi MF, Elkarrach K, Mechchate H, Conte R, Benlemlih M. Advanced Characterization of Organic Matter Decaying during Composting of Industrial Waste Using Spectral Methods. Processes. 2021; 9(8):1364. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9081364
Chicago/Turabian StyleBiyada, Saloua, Mohammed Merzouki, Hamada Imtara, Mohamed F. Alajmi, Karima Elkarrach, Hamza Mechchate, Raffaele Conte, and Mohamed Benlemlih. 2021. "Advanced Characterization of Organic Matter Decaying during Composting of Industrial Waste Using Spectral Methods" Processes 9, no. 8: 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9081364







