Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders
Abstract
:1. Introduction
- Profile the VOC composition of commercial southern Queensland ciders;
- Determine if VOC composition could be used as a means of discriminating between ciders produced by different manufacturers.
2. Materials and Methods
2.1. Reagents
2.2. Cider Samples
2.3. Extraction Procedure
2.4. VOC Analysis by GC–MS
2.5. Chemometric Analysis
3. Results and Discussion
3.1. Esters
3.2. Alcohols
3.3. Acids
3.4. Monoterpenes
3.5. Volatile Phenols
3.6. Ketones
3.7. Ethers
3.8. Furans
3.9. Acetal
3.10. Aldehydes
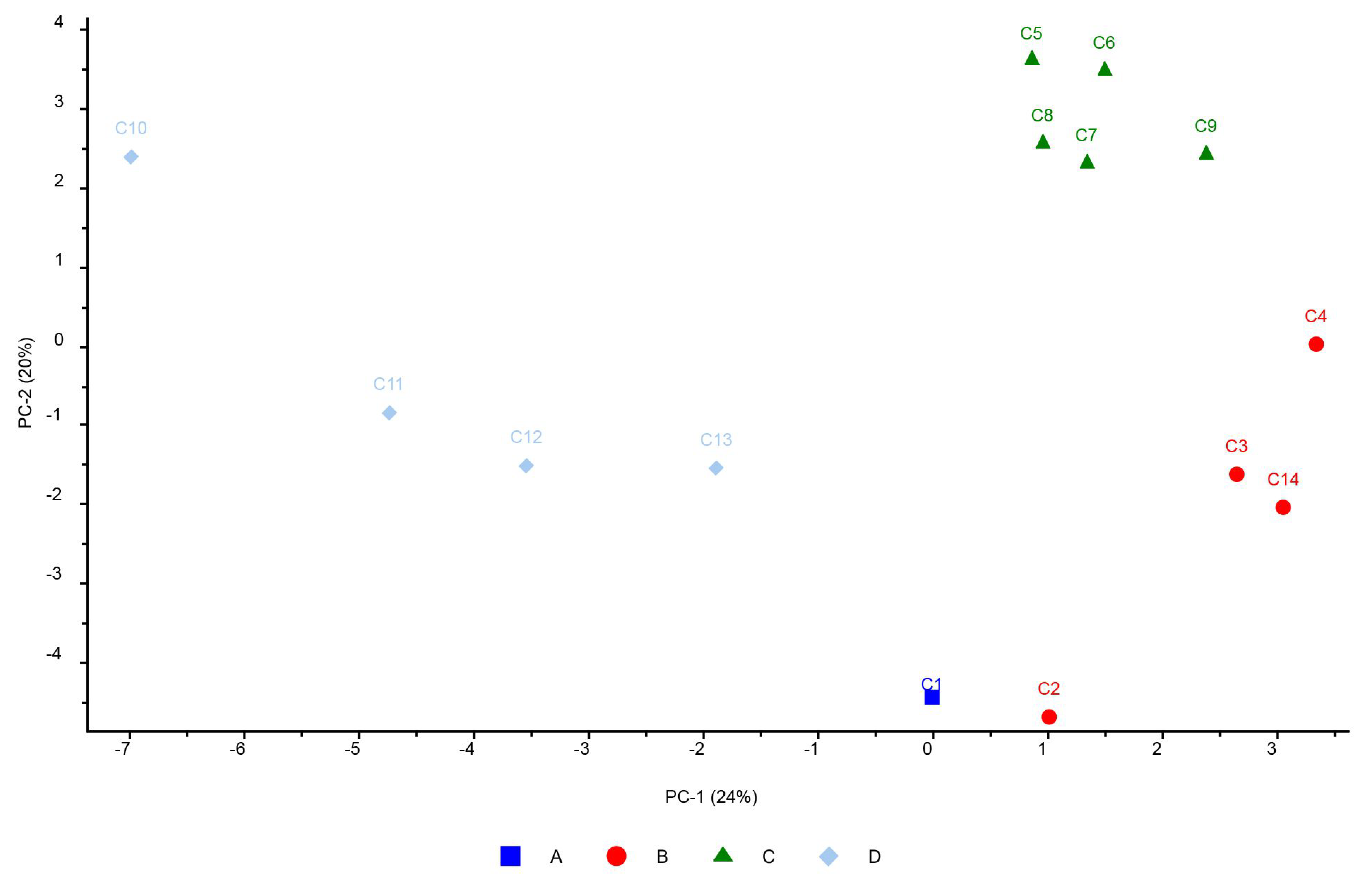
3.11. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, M. The Australian Cider Guide. Available online: http://www.cideraustralia.org.au/the-australian-cider-guide/ (accessed on 7 January 2021).
- Australian Bereau of Statistics. Apparent Consumption of Alcohol, Australia. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/apparent-consumption-alcohol-australia/latest-release (accessed on 7 January 2021).
- Cider Australia. Trust Mark. Available online: http://www.cideraustralia.org.au/trustmark/ (accessed on 7 January 2021).
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of aroma compounds in apple cider using solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Gonçalves, J.; Câmara, J.S. Effectiveness of high-throughput miniaturized sorbent-and solid phase microextraction techniques combined with gas chromatography–mass spectrometry analysis for a rapid screening of volatile and semi-volatile composition of wines—A comparative study. Talanta 2012, 88, 79–94. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Medina, S.; Pereira, R.; Camara, J.S. Untargeted fingerprinting of cider volatiles from different geographical regions by HS-SPME/GC-MS. Microchem. J. 2019, 148, 643–651. [Google Scholar] [CrossRef]
- Poll, L. Influence of storage temperature on sensory evaluation and composition of volatiles of McIntosh apple juice. LWT 1983, 16, 220–223. [Google Scholar]
- Poll, L. The influence of apple ripeness and juice storage temperature on the sensory evaluation and composition (volatile and non-volatile components) of apple juice. Lebensm. Wiss. Technol. 1985, 18, 205–211. [Google Scholar]
- Cunningham, D.; Acree, T.; Barnard, J.; Butts, R.M.; Braell, P. Charm analysis of apple volatiles. Food Chem. 1986, 19, 137–147. [Google Scholar] [CrossRef]
- Petró-Turza, M.; Szarfoldi-Szalma, I.; Madarassy-Mersich, E.; Teleky-Vámossy, G.; Fuzesi-Kardos, K. Correlation between chemical composition and sensory quality of natural apple aroma condensates. Die Nahr. Food 1986, 30, 765. [Google Scholar]
- Young, H.; Gilbert, J.M.; Murray, S.H.; Ball, R.D. Causal effects of aroma compounds on Royal Gala apple flavours. J. Sci. Food Agric. 1996, 71, 329–336. [Google Scholar] [CrossRef]
- López, M.; Lavilla, M.; Riba, M.; Vendrell, M. Comparison of volatile compounds in two seasons in apples: Golden Delicious and Granny Smith. J. Food Qual. 1998, 21, 155–166. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Changes in the profile of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Moriguchi, S.; Fukuda, H.; Ichimura, N.; Kato, Y.; Banba, Y. Composition of volatile compounds of apple fruits in relation to cultivars. J. Jpn. Soc. Hortic. Sci. 1986, 55, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Holland, D.; Larkov, O.; Bar-Ya’akov, I.; Bar, E.; Zax, A.; Brandeis, E.; Ravid, U.; Lewinsohn, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Villatoro, C.; Luisa López, M.; Echeverria, G.; Graell, J. Effect of controlled atmospheres and shelf life period on concentrations of volatile substances released by ‘Pink Lady®’apples and on consumer acceptance. J. Sci. Food Agric. 2009, 89, 1023–1034. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Pereira, R.; Câmara, J.S. Evaluation of Volatilomic Fingerprint from Apple Fruits to Ciders: A Useful Tool to Find Putative Biomarkers for Each Apple Variety. Foods 2020, 9, 1830. [Google Scholar] [CrossRef]
- Nešpor, J.; Karabín, M.; Štulíková, K.; Dostálek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019, 24, 2117. [Google Scholar] [CrossRef] [Green Version]
- Villière, A.; Arvisenet, G.; Bauduin, R.; Le Quéré, J.M.; Sérot, T. Influence of cider-making process parameters on the odourant volatile composition of hard ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe strains on chemical composition and sensory quality of ciders made from Finnish apple cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef]
- Nogueira, A.; Mongruel, C.; Simões, D.R.S.; Waszczynskyj, N.; Wosiacki, G. Effect of biomass reduction on the fermentation of cider. Braz. Arch. Biol. Technol. 2007, 50, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Madrera, R.; Picinelli Lobo, A.; Mangas Alonso, J.J. Effect of cider maturation on the chemical and sensory characteristics of fresh cider spirits. Food Res. Int. 2010, 43, 70–78. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B.; Picinelli Lobo, A. Chemical and sensory changes in fresh cider spirits during maturation in inert containers. J. Sci. Food Agric. 2011, 91, 797–804. [Google Scholar] [CrossRef] [PubMed]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Cozzolino, D.; Power, A.; Chapman, J. Interpreting and reporting principal component analysis in food science analysis and beyond. Food Anal. Methods 2019, 12, 2469–2473. [Google Scholar] [CrossRef]
- Echeverría, G.; Graell, J.; Lara, I.; López, M. Physicochemical measurements in ‘Mondial Gala®’apples stored at different atmospheres: Influence on consumer acceptability. Postharvest Biol. Technol. 2008, 50, 135–144. [Google Scholar] [CrossRef]
- Echeverrıa, G.; Fuentes, T.; Graell, J.; Lara, I.; López, M. Aroma volatile compounds of ‘Fuji’apples in relation to harvest date and cold storage technology: A comparison of two seasons. Postharvest Biol. Technol. 2004, 32, 29–44. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of volatile and sensory profiles of apple juices to trace fruit origins and investigation of the relationship between the aroma properties and volatile constituents. LWT 2020, 124, 109203. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Propyl Propionate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7803 (accessed on 17 February 2021).
- Simpson, R.F. Volatile aroma components of Australian port wines. J. Sci. Food Agric. 1980, 31, 214–222. [Google Scholar] [CrossRef]
- Saerens, S.; Delvaux, F.; Verstrepen, K.; Van Dijck, P.; Thevelein, J.; Delvaux, F. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Nicolini, G.; Román, T.; Carlin, S.; Malacarne, M.; Nardin, T.; Bertoldi, D.; Larcher, R. Characterisation of single-variety still ciders produced with dessert apples in the Italian Alps. J. Inst. Brew. 2018, 124, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Decloux, M.; Dechatre, J.-C.; Legendre, P.; Guichard, H. Double batch cider distillation: Influence of the recycling of the separated fractions. LWT 2021, 146, 111420. [Google Scholar] [CrossRef]
- Mangwanda, T.; Johnson, J.B.; Mani, J.S.; Jackson, S.; Chandra, S.; McKeown, T.; White, S.; Naiker, M. Processes, Challenges and Optimisation of Rum Production from Molasses—A Contemporary Review. Fermentation 2021, 7, 21. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Lethuaut, L.; Prost, C.; Sérot, T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC–MS–O and GC× GC–TOF-MS. Food Chem. 2012, 131, 1561–1568. [Google Scholar] [CrossRef]
- Jordán, M.J.; Margaría, C.A.; Shaw, P.E.; Goodner, K.L. Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2003, 51, 1421–1426. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Chastrette, M.; Heintz, M.; Druilhe, A.; Lefort, D. Analyse chromatographique d’esters aliphatiques saturés. Relations rétention-structure et prévision de la rétention. Bull. Soc. Chim. Fr. 1974, 375, 1852–1856. [Google Scholar]
- Fan, S.; Chang, J.; Zong, Y.; Hu, G.; Jia, J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum Var. Aromaticum using three extraction methods and two columns. Molecules 2018, 23, 576. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, X.l.; Zhou, G.h. Comparative study of volatile compounds in traditional Chinese Nanjing marinated duck by different extraction techniques. Int. J. Food Sci. Technol. 2007, 42, 543–550. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Technische Universität München: Munich, Germany, 1998; pp. 1–63. [Google Scholar]
- Mevy, J.-P.; Bessiere, J.-M.; Greff, S.; Zombre, G.; Viano, J. Composition of the volatile oil from the leaves of Ximenia americana L. Biochem. Syst. Ecol. 2006, 34, 549–553. [Google Scholar] [CrossRef]
- Jordan, M.J.; Margaria, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef]
- El-Sayed, A.; Heppelthwaite, V.; Manning, L.; Gibb, A.; Suckling, D. Volatile constituents of fermented sugar baits and their attraction to lepidopteran species. J. Agric. Food Chem. 2005, 53, 953–958. [Google Scholar] [CrossRef]
- Bruna, J.M.; Hierro, E.M.; de la Hoz, L.; Mottram, D.S.; Fernández, M.; Ordóñez, J.A. Changes in selected biochemical and sensory parameters as affected by the superficial inoculation of Penicillium camemberti on dry fermented sausages. Int. J. Food Microbiol. 2003, 85, 111–125. [Google Scholar] [CrossRef]
- Machiels, D.; Van Ruth, S.M.; Posthumus, M.A.; Istasse, L. Gas chromatography-olfactometry analysis of the volatile compounds of two commercial Irish beef meats. Talanta 2003, 60, 755–764. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G. Potent aroma compounds in Parmigiano Reggiano cheese studied using a dynamic headspace (purge-trap) method. Flavour Fragr. J. 2003, 18, 252–259. [Google Scholar] [CrossRef]
- Choi, H.-S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov× C. sinensis Osbeck)× C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef]
- Pino, J.; Marbot, R.; Rosado, A. Volatile constituents of star apple (Chrysophyllum cainito L.) from Cuba. Flavour Fragr. J. 2002, 17, 401–403. [Google Scholar] [CrossRef]
- da Silva, M.H.L.; Andrade, E.H.A.; Zoghbi, M.d.G.B.; Luz, A.I.R.; da Silva, J.D.; Maia, J.G.S. The essential oils of Lantana camara L. occurring in North Brazil. Flavour Fragr. J. 1999, 14, 208–210. [Google Scholar] [CrossRef]
- Lalel, H.J.; Singh, Z.; Tan, S.C. Glycosidically-bound aroma volatile compounds in the skin and pulp of ‘Kensington Pride’mango fruit at different stages of maturity. Postharvest Biol. Technol. 2003, 29, 205–218. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J. Synthesis of higher alcohols during cider processing. Food Chem. 1999, 67, 287–294. [Google Scholar] [CrossRef]
- Bingman, M.T.; Stellick, C.E.; Pelkey, J.P.; Scott, J.M.; Cole, C.A. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages 2020, 6, 40. [Google Scholar] [CrossRef]
- Azhu Valappil, Z.; Fan, X.; Zhang, H.Q.; Rouseff, R.L. Impact of thermal and nonthermal processing technologies on unfermented apple cider aroma volatiles. J. Agric. Food Chem. 2009, 57, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Hubert, B.; Baron, A.; Le Quere, J.-M.; Renard, C.M. Influence of prefermentary clarification on the composition of apple musts. J. Agric. Food Chem. 2007, 55, 5118–5122. [Google Scholar] [CrossRef] [PubMed]
- Beech, F.; Carr, J. Cider and perry. Alcohol. Beverages 1977, 1, 139–313. [Google Scholar]
- Mei, J.; Min, H.; Lü, Z. Enhanced biotransformation of L-phenylalanine to 2-phenylethanol using an in situ product adsorption technique. Process Biochem. 2009, 44, 886–890. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Han, Y. Quantification of volatile compounds in Chinese ciders by stir bar sorptive extraction (SBSE) and gas chromatography–mass spectrometry (GC-MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. Lwt Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Etschmann, M.; Schrader, J. An aqueous–organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl. Microbiol. Biotechnol. 2006, 71, 440–443. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.A.; Bredie, W.L. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef]
- Bult, J.H.; Schifferstein, H.N.; Roozen, J.P.; Boronat, E.D.; Voragen, A.G.; Kroeze, J.H. Sensory evaluation of character impact components in an apple model mixture. Chem. Senses 2002, 27, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Eleutério dos Santos, C.M.; Pietrowski, G.d.A.M.; Braga, C.M.; Rossi, M.J.; Ninow, J.; Machado dos Santos, T.P.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple aminoacid profile and yeast strains in the formation of fusel alcohols and esters in cider production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B. Determination of volatile compounds in cider spirits by gas chromatography with direct injection. J. Chromatogr. Sci. 2007, 45, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT 2020, 124, 109184. [Google Scholar] [CrossRef]
- Tao, Y.-S.; Li, H. Active volatiles of cabernet sauvignon wine from Changli County. Health 2009, 1, 176. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Madrera, R.; Mangas Alonso, J. Distribution of the principal minor volatiles during cider distillation in ‘alquitara’. Acta Aliment. 2011, 40, 262–269. [Google Scholar] [CrossRef] [Green Version]
- De Torres, C.; Díaz-Maroto, M.; Hermosín-Gutiérrez, I.; Pérez-Coello, M. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Leon-Ruiz, V.; Alañon, M.; Pérez-Coello, M.; González-Porto, A. Floral origin markers for authenticating Lavandin honey (Lavandula angustifolia x latifolia). Discrimination from Lavender honey (Lavandula latifolia). Food Control. 2014, 37, 362–370. [Google Scholar] [CrossRef]
- Leão, K.M.; Sampaio, K.L.; Pagani, A.A.; Da Silva, M.A.A. Odor potency, aroma profile and volatiles composition of cold pressed oil from industrial passion fruit residues. Ind. Crop. Prod. 2014, 58, 280–286. [Google Scholar] [CrossRef]
- Connell, D.; Sutherland, M. A re-examination of gingerol, shogaol, and zingerone, the pungent principles of ginger (Zingiber officinale Roscoe). Aust. J. Chem. 1969, 22, 1033–1043. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Ye, P.; Lin, F.; Huang, J.; Wang, H.; Zhou, R.; Zhang, S.; Zhou, J.; Cai, L. Characterization of major properties and aroma profile of kiwi wine co-cultured by Saccharomyces yeast (S. cerevisiae, S. bayanus, S. uvarum) and T. delbrueckii. Eur. Food Res. Technol. 2020, 246, 807–820. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Xu, Y. Comparison on aroma compounds in Chinese soy sauce and strong aroma type liquors by gas chromatography–olfactometry, chemical quantitative and odor activity values analysis. Eur. Food Res. Technol. 2014, 239, 813–825. [Google Scholar] [CrossRef]
- Alexandre, H.; Charpentier, C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Lino, C.; Pena, A. Occurrence of caffeine, saccharin, benzoic acid and sorbic acid in soft drinks and nectars in Portugal and subsequent exposure assessment. Food Chem. 2010, 121, 503–508. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and occurrence of 1, 8-cineole (Eucalyptol) in Australian wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Herve, E.; Price, S.; Burns, G. Eucalyptol in wines showing a “eucalyptus” aroma. In Proceedings of the VIIeme Symp. Internat. d’Oenologie, Actualites Oenologiques, Bordeaux, France, 19–21 June 2003; pp. 19–21. [Google Scholar]
- Walia, M.; Mann, T.S.; Kumar, D.; Agnihotri, V.K.; Singh, B. Chemical composition and in vitro cytotoxic activity of essential oil of leaves of Malus domestica growing in Western Himalaya (India). Evid. Based Complementary Altern. Med. 2012, 2012, 649727. [Google Scholar] [CrossRef] [Green Version]
- Wohlmuth, H.; Smith, M.K.; Brooks, L.O.; Myers, S.P.; Leach, D.N. Essential oil composition of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) grown in Australia. J. Agric. Food Chem. 2006, 54, 1414–1419. [Google Scholar] [CrossRef]
- Alegre, Y.; Sáenz-Navajas, M.-P.; Ferreira, V.; García, D.; Razquin, I.; Hernández-Orte, P. Rapid strategies for the determination of sensory and chemical differences between a wealth of similar wines. Eur. Food Res. Technol. 2017, 243, 1295–1309. [Google Scholar] [CrossRef]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Januszek, M.; Satora, P.; Tarko, T. Oenological characteristics of fermented apple musts and volatile profile of brandies obtained from different apple cultivars. Biomolecules 2020, 10, 853. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Buettner, A. Influence of the chemical structure on the odor characters of β-citronellol and its oxygenated derivatives. Food Chem. 2017, 232, 704–711. [Google Scholar] [CrossRef]
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coïsson, J.D.; Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef]
- Gamero, A.; Manzanares, P.; Querol, A.; Belloch, C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int. J. Food Microbiol. 2011, 145, 92–97. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Carbó, N.; Costelli, P.; Baccino, F.M.; López-Soriano, F.J.; Argilés, J.M. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem. Biophys. Res. Commun. 1999, 254, 739–743. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Picinelli Lobo, A.; Suárez Valles, B. Phenolic profile of Asturian (Spain) natural cider. J. Agric. Food Chem. 2006, 54, 120–124. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The mouthfeel of white wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef]
- Clifford, A.J.; Ebeler, S.E.; Ebeler, J.D.; Bills, N.D.; Hinrichs, S.H.; Teissedre, P.-L.; Waterhouse, A.L. Delayed tumor onset in transgenic mice fed an amino acid–based diet supplemented with red wine solids. Am. J. Clin. Nutr. 1996, 64, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Liu, Y.; He, Q.; Liu, P.; Che, Z.; Wang, X.; Huang, J. Characterization of odor components of Pixian Douban (broad bean paste) by aroma extract dilute analysis and odor activity values. Int. J. Food Prop. 2019, 22, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Palacios, A.; Suárez, C.; Krieger, S.; Théodore, D.; Otaño, L.; Peña, F. Perception by wine drinkers of sensory defects caused by uncontrolled malolactic fermentation. In Wine Quality and Malolactic Fermentation, Proceedings of the XVIth Entretiens Scientifiques Lallemand, Porto, Portugal, 4–5 May 2004; Lallemand: Montreal, QC, Canada, 2004; pp. 45–52. [Google Scholar]
- Antón-Díaz, M.J.; Valles, B.S.; Mangas-Alonso, J.J.; Fernández-García, O.; Picinelli-Lobo, A. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chem. 2016, 190, 1116–1122. [Google Scholar] [CrossRef]
- Claisse, O.; Lonvaud-Funel, A. Assimilation of glycerol by a strain of Lactobacillus collinoides isolated from cider. Food Microbiol. 2000, 17, 513–519. [Google Scholar] [CrossRef]
- Laplace, J.; Sauvageot, N.; Hartke, A.; Auffray, Y. Characterization of Lactobacillus collinoides response to heat, acid and ethanol treatments. Appl. Microbiol. Biotechnol. 1999, 51, 659–663. [Google Scholar] [CrossRef]
- Zhao, D.; Barrientos, J.U.; Wang, Q.; Markland, S.M.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W.; Kniel, K.E.; Moraru, C.I. Efficient reduction of pathogenic and spoilage microorganisms from apple cider by combining microfiltration with UV treatment. J. Food Prot. 2015, 78, 716–722. [Google Scholar] [CrossRef]
- Versini, G.; Franco, M.A.; Moser, S.; Barchetti, P.; Manca, G. Characterisation of apple distillates from native varieties of Sardinia island and comparison with other Italian products. Food Chem. 2009, 113, 1176–1183. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Saftner, R.A. Temperature-dependent autoxidation of conjugated trienols from apple peel yields 6-methyl-5-hepten-2-one, a volatile implicated in induction of scald. J. Agric. Food Chem. 2000, 48, 2040–2043. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Diacetone Alcohol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diacetone-alcohol (accessed on 9 March 2021).
- Yong, Y.; Wenyi, T. Head-space solid phase micro-extraction followed by GC/MS analysis of the volatile components in seeds of Cinnamonum camphora. Am. J. Biochem. Biotechnol. 2005, 1, 173–175. [Google Scholar] [CrossRef]
- Ibrahim, H.; Nwanya, K.; Ayilara, S.; Adegbola, O.; Nwakuba, D.; Tyoor, A.; Ba’are, A.; Shuaibu, H. Potential of earleaf acacia (Acacia auriculiformis) leaves for industrial raw materials. Int. J. Sci. Eng. Appl. Sci. 2015, 1, 462–467. [Google Scholar]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abalos, D.; Vejarano, R.; Morata, A.; González, C.; Suárez-Lepe, J.A. The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. Eur. Food Res. Technol. 2011, 232, 663–669. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Blanco Gomis, D.; Mangas Alonso, J.J. Influence of distillation system, oak wood type, and aging time on composition of cider brandy in phenolic and furanic compounds. J. Agric. Food Chem. 2003, 51, 7969–7973. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Hogg, T.; Silva, M. Application of a liquid chromatographic method for the determination of phenolic compounds and furans in fortified wines. Food Chem. 1999, 64, 115–122. [Google Scholar] [CrossRef]
- Rusu, E.; Obadă, L.; Dumanova, V.; Cibuc, M. Aromatic compounds of wine obtained from the new variety of domestic selection Viorica. In Proceedings of the Modern Technologies in the Food Industry, Chișinău, Moldova, 1–3 November 2012; pp. 115–120. [Google Scholar]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta 2008, 621, 79–85. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Januszek, M. Effect of Musts Oxygenation at Various Stages of Cider Production on Oenological Parameters, Antioxidant Activity, and Profile of Volatile Cider Compounds. Biomolecules 2020, 10, 890. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Yang, B. Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol. 2019, 80, 25–39. [Google Scholar] [CrossRef]
- Deot, P. Presence and Growth of Microorganisms in Iowa Apple Cider. Master’s Thesis, Iowa State University, Ames, Iowa, 2003. [Google Scholar]
- Janzantti, N.S.; Monteiro, M. Changes in the aroma of organic passion fruit (Passiflora edulis Sims f. flavicarpa Deg.) during ripeness. Lwt Food Sci. Technol. 2014, 59, 612–620. [Google Scholar] [CrossRef] [Green Version]



| Cider | Manufacturer | Region of Manufacture | Description from Label | Manufacturing Information | Ethanol Content (%) |
|---|---|---|---|---|---|
| 1 | A | Stanthorpe, QLD | Medium dry, hazy | 70/30% red/green apples. Champagne yeast used | 4.8 |
| 2 | B | Sunshine Coast, QLD | Medium dry | - | 4.8 |
| 3 | B | Sunshine Coast, QLD | Bone dry, fruit with a honey note, wine-like | - | 7.0 |
| 4 | B | Sunshine Coast, QLD | Bone dry, acidic, yeasty | Bottle fermented | 7.2 |
| 5 | C | Bundaberg, QLD | Contains ginger and orange | - | 8.0 |
| 6 | C | Bundaberg, QLD | Contains passionfruit | - | 8.0 |
| 7 | C | Bundaberg, QLD | Dry | Royal Gala, Fuji, and Granny Smith apples ^ | 6.2 |
| 8 | C | Bundaberg, QLD | Sweet | Royal Gala and Red Delicious apples ^ | 6.1 |
| 9 | C | Bundaberg, QLD | Contains grape | - | 8.0 |
| 10 | D | Tamborine Mountain, QLD | Botanical, bright, spicy; contains ginger | - | 5.0 |
| 11 | D | Tamborine Mountain, QLD | Contains shiraz wine | - | 5.0 |
| 12 | D | Tamborine Mountain, QLD | Crisp, juicy | Made from Pink Lady apples ^ | 5.2 |
| 13 | D | Tamborine Mountain, QLD | Dry, champagne-like | Made from Granny Smith apples ^ | 5.9 |
| 14 | B | Sunshine Coast, QLD | Dry, sparkling | Bottle fermented, champagne method | 8.5 |
| Chemical Class Based on Functional Group | Number of Individual Compounds | Percentage of Volatile Diversity |
|---|---|---|
| Esters | 8 | 20 |
| Alcohols | 7 | 17.5 |
| Acids | 6 | 15 |
| Monoterpenes | 6 | 15 |
| Volatile phenols | 5 | 12.5 |
| Ketones | 3 | 7.5 |
| Furans | 2 | 5 |
| Ethers | 1 | 2.5 |
| Acetals | 1 | 2.5 |
| Aldehydes | 1 | 2.5 |
| Total | 40 | 100 |
| No. | Retention Time | Compound | Chemical Class | Ident. Method | LRI ^ | Lit. LRI ^ | Cider Sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |||||||
| 1 | 2.29 | Diethyl acetal | Acetal | MS, LRI | 756 | 734 [38] | 12.9 | 6.0 | 14.5 | 7.5 | 6.6 | 5.9 | 4.8 | 4.1 | 5.8 | 7.6 | 12.2 | 13.8 | 9.5 | 4.7 |
| 2 | 2.50 | Butanoic acid | Acid | MS, LRI | 766 | 775 * | - | - | - | - | - | - | - | - | - | 0.7 | 0.9 | 0.6 | 0.2 | - |
| 3 | 2.56 | Propyl propionate | Ester | MS, LRI | 769 | 785 * | 2.9 | 5.0 | 0.4 | - | 1.2 | 1.1 | 1.8 | 1.3 | 2.4 | 2.1 | 5.5 | 3.6 | 2.0 | 0.4 |
| 4 | 2.79 | 2,3-Butanediol | Alcohol | LRI | 779 | 770 [39] | 0.5 | 0.6 | 1.1 | - | - | - | - | - | - | 0.4 | 0.8 | 0.6 | 0.4 | - |
| 5 | 2.88 | 3-Hexen-2-one | Ketone | MS, LRI | 783 | 762 * | 5.3 | 5.0 | 9.8 | 10.7 | 11.0 | 7.9 | 8.1 | 7.8 | 10.1 | 6.2 | 7.2 | 7.2 | 7.0 | 7.0 |
| 6 | 3.09 | 3-Methoxy-1-butanol | Alcohol | MS, LRI | 792 | 773 * | 0.5 | 0.6 | 1.4 | 1.4 | 1.5 | 1.2 | 1.2 | 1.1 | 1.4 | 0.7 | 1.0 | 1.0 | 1.0 | 0.9 |
| 7 | 3.16 | Isopropyl 2-methylpropanoate | Ester | LRI | 796 | 780 [40] | 2.4 | 3.1 | 9.6 | 6.7 | 4.5 | 3.3 | 3.3 | 3.2 | 4.1 | 2.5 | 3.0 | 3.1 | 3.0 | 2.9 |
| 8 | 3.62 | Furfural | Furan | MS, LRI | 819 | 830 [39] | 0.6 | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 9 | 3.73 | 3-Ethoxy-1-propanol | Alcohol | MS, LRI | 825 | 837 * | 0.2 | - | - | - | 0.4 | 0.4 | 0.8 | 0.6 | 0.6 | 1.8 | 3.8 | 1.6 | 2.6 | - |
| 10 | 3.81 | Diacetone alcohol | Ketone | MS, LRI | 829 | 829 [41] | 0.3 | 0.3 | 1.2 | 0.9 | 1.1 | 0.9 | 1.0 | 0.8 | 1.3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.8 |
| 11 | 4.40 | 1-Hexanol | Alcohol | MS, LRI | 859 | 867 [39] | 1.7 | 1.3 | 1.7 | 0.5 | 0.8 | 1.9 | 2.0 | 1.5 | 2.2 | 0.6 | 4.9 | 7.7 | 0.9 | 1.0 |
| 12 | 4.55 | Pentyl acetate | Ester | MS, LRI | 867 | 884 * | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 | 0.4 | 0.2 | 0.2 | 0.1 | 1.4 | 2.0 | 1.9 | 1.2 | - |
| 13 | 5.27 | 4-Hydroxy-butanoic acid | Acid | MS, LRI | 904 | 933 [42] | - | 0.8 | 0.4 | - | - | - | - | - | - | 0.1 | - | - | 0.2 | - |
| 14 | 6.43 | Benzaldehyde | Aldehyde | MS, LRI | 959 | 950 [41] | - | - | - | - | - | 1.3 | - | - | - | - | - | - | - | - |
| 15 | 6.67 | Hexanoic acid | Acid | MS, LRI | 970 | 1019 [43] | 1.1 | 0.2 | 1.2 | 0.3 | 0.9 | 1.2 | 1.6 | 2.1 | 0.8 | 4.8 | 5.4 | 4.3 | 5.2 | - |
| 16 | 6.78 | Methionol | Alcohol | MS, LRI | 975 | 978 [43] | 0.6 | 0.8 | 0.4 | 0.7 | - | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | - | - | 0.6 |
| 17 | 6.91 | 6-Methyl-5-hepten-2-one | Ketone | MS, LRI | 981 | 966 [44] | - | - | 0.2 | - | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.9 | 0.3 | - | 0.2 | 0.2 |
| 18 | 7.21 | Ethyl hexanoate | Ester | MS, LRI | 996 | 997 [45] | 0.3 | - | 0.3 | 0.3 | 0.6 | 0.5 | 0.7 | 2.1 | 0.2 | 0.9 | 1.1 | 0.9 | 1.0 | - |
| 19 | 7.26 | 1,4-Diethoxy-2-butene | Ether | MS | 998 | 976 * | 0.4 | 0.3 | 0.3 | 0.4 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.5 | 0.5 | 0.4 | 0.6 | 0.4 |
| 20 | 7.99 | Eucalyptol | Terpenoid | MS, LRI | 1032 | 1026 [41] | - | - | - | - | 1.2 | - | - | - | - | 8.5 | 0.2 | - | - | - |
| 21 | 8.00 | Benzyl alcohol | Alcohol | MS, LRI | 1032 | 1032 [46] | - | - | - | - | - | 7.9 | - | - | - | - | - | - | - | - |
| 22 | 8.78 | Sorbic acid | Acid | MS, LRI | 1070 | 1045 [47] | - | - | - | - | 25.9 | 30.8 | 47.8 | 52.3 | 49.9 | - | - | - | - | - |
| 23 | 9.44 | Linalool | Terpenoid | MS, LRI | 1099 | 1090 [41] | - | 0.4 | 0.3 | 0.7 | 2.1 | 0.9 | 0.4 | 0.4 | 0.3 | 2.1 | 0.7 | 0.4 | 0.7 | 0.2 |
| 24 | 9.51 | Dihydromyrcenol | Terpenoid | LRI | 1102 | 1080 * | 0.2 | 0.3 | 0.6 | 0.5 | 0.5 | 0.3 | 0.4 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | |
| 25 | 9.71 | 2-Phenylethanol | Alcohol | MS, LRI | 1112 | 1110 [46] | 54.9 | 59.8 | 35.1 | 52.7 | 25.0 | 19.1 | 14.8 | 13.3 | 12.9 | 28.4 | 27.7 | 23.9 | 32.3 | 60.3 |
| 26 | 10.65 | Benzoic acid | Acid | MS, LRI | 1156 | 1171 [48] | - | - | - | - | - | 1.0 | - | - | - | - | - | 1.9 | - | - |
| 27 | 10.79 | 2-Ethylphenol | Phenol | MS, LRI | 1162 | 1169 [43] | 0.6 | 5.3 | 6.1 | 1.1 | - | - | - | - | - | - | 1.2 | 0.9 | 6.8 | 4.9 |
| 28 | 10.87 | Octanoic acid | Acid | MS, LRI | 1166 | 1179 [45] | 2.2 | 0.7 | 2.7 | 3.8 | 5.8 | 2.7 | 4.3 | 3.4 | 2.1 | 10.1 | 9.5 | 3.9 | 4.1 | - |
| 29 | 11.03 | Endo-Borneol | Terpenoid | MS, LRI | 1174 | 1157 [41] | - | - | - | - | 0.6 | - | - | - | - | 1.4 | - | - | - | - |
| 30 | 11.08 | Diethyl succinate | Ester | MS, LRI | 1176 | 1179 [39] | - | 1.2 | 3.9 | 3.7 | 1.1 | 0.7 | 0.2 | - | 0.2 | - | 0.3 | 0.1 | 1.1 | 4.9 |
| 31 | 11.47 | Ethyl octanoate | Ester | MS, LRI | 1195 | 1195 [49] | - | - | 0.5 | 0.3 | - | 0.2 | 0.6 | 0.4 | 0.2 | - | 0.5 | 0.4 | 0.5 | - |
| 32 | 11.50 | Alpha-terpineol | Terpenoid | MS, LRI | 1196 | 1185 [50] | - | - | - | 1.3 | 3.2 | 0.2 | - | - | - | 4.0 | - | - | 3.2 | - |
| 33 | 11.89 | Coumarin | Furan | MS, LRI | 1215 | 1224 [51] | 0.9 | 0.2 | 0.3 | - | 0.6 | 0.5 | 0.5 | 0.7 | 0.4 | 0.3 | 0.3 | 0.7 | 0.5 | - |
| 34 | 12.10 | Citronellol | Terpenoid | MS, LRI | 1226 | 1228 [39] | - | - | - | - | - | - | - | - | - | 1.1 | 0.2 | - | - | - |
| 35 | 12.60 | Chavicol | Phenol | MS, LRI | 1250 | 1253 [39] | 0.2 | 1.0 | 1.0 | 0.7 | 0.6 | 4.2 | 2.2 | 2.0 | 1.1 | 1.9 | 2.6 | 1.3 | - | 0.5 |
| 36 | 12.78 | Hexyl 2-methylbutanoate | Ester | MS, LRI | 1259 | 1236 [52] | 5.9 | 2.1 | - | - | - | - | 0.6 | 0.2 | 1.6 | 4.5 | 4.0 | 18.3 | - | - |
| 37 | 12.82 | Diethyl maleate | Ester | MS, LRI | 1261 | 1313 * | 0.4 | 1.1 | 4.4 | 2.6 | 2.9 | 2.4 | 0.9 | 0.9 | 1.0 | 2.3 | 0.6 | - | 14.2 | 8.6 |
| 38 | 14.62 | Eugenol | Phenol | MS, LRI | 1353 | 1340 [41] | - | 0.8 | 0.4 | 0.5 | 0.2 | 1.4 | 0.7 | 0.5 | 0.3 | 0.6 | 1.1 | - | - | - |
| 39 | 15.90 | Tyrosol | Phenol | MS, LRI | 1421 | 1356 * | 4.6 | 3.3 | 2.3 | 2.4 | 0.6 | 0.7 | 0.5 | - | - | 1.3 | 1.5 | 0.9 | 1.1 | 1.6 |
| 40 | 19.73 | Zingerone | Phenol | MS, LRI | 1641 | 1653 [53] | - | - | - | - | 0.5 | - | - | - | - | 1.6 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, A.; Johnson, J.B.; Batley, R.; Lal, P.; Wakeling, L.; Naiker, M. Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders. Beverages 2021, 7, 28. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020028
Wilson A, Johnson JB, Batley R, Lal P, Wakeling L, Naiker M. Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders. Beverages. 2021; 7(2):28. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020028
Chicago/Turabian StyleWilson, Arron, Joel B. Johnson, Ryan Batley, Pawan Lal, Lara Wakeling, and Mani Naiker. 2021. "Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders" Beverages 7, no. 2: 28. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020028