Advanced Drug Delivery Systems and Technology in Hungary
Share This Topical Collection
Editors
 Prof. Dr. Romána Zelkó
Prof. Dr. Romána Zelkó
 Prof. Dr. Romána Zelkó
Prof. Dr. Romána Zelkó
E-Mail
Website
Guest Editor
University Pharmacy Department of Pharmacy Administration, Semmelweis University, Hőgyes Endre utca 7-9, H-1092 Budapest, Hungary
Interests: polymeric delivery systems; physical ageing of polymers; solid-state characterisation; functionality-related characteristics of polymeric delivery systems; microstructural characterisation of dosage forms; stability tracking of solid dosage forms; regulatory aspects of dosage forms
Special Issues, Collections and Topics in MDPI journals
 Prof. Dr. Istvan Antal
Prof. Dr. Istvan Antal
 Prof. Dr. Istvan Antal
Prof. Dr. Istvan Antal
E-Mail
Website
Guest Editor
Department of Pharmaceutics, Semmelweis University, Hőgyes Endre u. 7, H-1092 Budapest, Hungary
Interests: drug delivery systems; formulation design; multiparticulates; microparticles; nanocarriers; modified drug release; dissolution improvement; bioavailability enhancement; excipient applications
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
As a result of intensive research and development activities in recent decades, advanced drug-delivery systems have emerged. These formulations allow the predictability and optimisation of therapeutic effects by enabling the production of continuous or intermittent, accelerated, extended, delayed, externally controlled or self-regulated drug-delivery systems with a wide range of safe variable-release profiles, drug-release mechanisms and programmability.
This Special Issue highlights and captures the recent progress in drug delivery within the traditionally acknowledged Hungarian academic and pharmaceutical industrial context.
We invite articles on all aspects of drug-delivery sciences, from preclinical formulation development to human clinical trials, which bring to light the world-class research currently undertaken in Hungary for this Special Issue.
Prof. Dr. Romána Zelkó
Prof. Dr. Istvan Antal
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- drug delivery
- advanced technologies
- drug development
- formulation and dosage form development
- translational research
- biologicals
- small molecules
- clinical trials
- pharmacokinetics
- medical devices
- route of administration
Related Special Issue
Published Papers (16 papers)
Open AccessReview
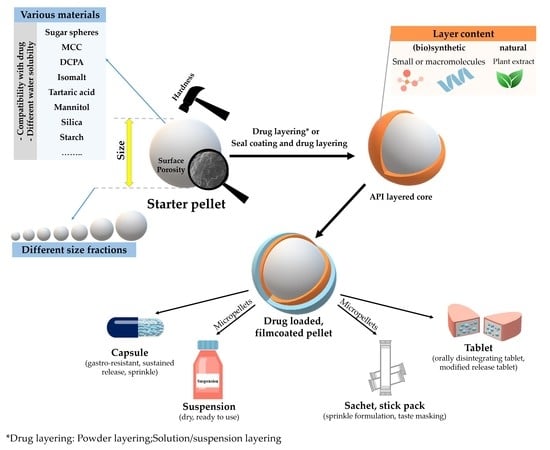
Review on Starter Pellets: Inert and Functional Cores
by
Nikolett Kállai-Szabó, Miléna Lengyel, Dóra Farkas, Ádám Tibor Barna, Christian Fleck, Bálint Basa and István Antal
Cited by 3 | Viewed by 7434
Abstract
A significant proportion of pharmaceuticals are now considered multiparticulate systems. Modified-release drug delivery formulations can be designed with engineering precision, and patient-centric dosing can be accomplished relatively easily using multi-unit systems. In many cases, Multiple-Unit Pellet Systems (MUPS) are formulated on the basis
[...] Read more.
A significant proportion of pharmaceuticals are now considered multiparticulate systems. Modified-release drug delivery formulations can be designed with engineering precision, and patient-centric dosing can be accomplished relatively easily using multi-unit systems. In many cases, Multiple-Unit Pellet Systems (MUPS) are formulated on the basis of a neutral excipient core which may carry the layered drug surrounded also by functional coating. In the present summary, commonly used starter pellets are presented. The manuscript describes the main properties of the various nuclei related to their micro- and macrostructure. In the case of layered pellets formed based on different inert pellet cores, the drug release mechanism can be expected in detail. Finally, the authors would like to prove the industrial significance of inert cores by presenting some of the commercially available formulations.
Full article
►▼
Show Figures
Open AccessArticle
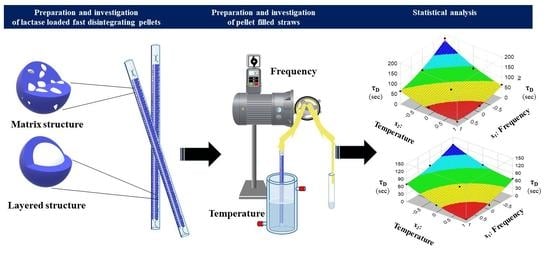
Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw
by
Márton Király, Konrád Sántha, Barnabás Kállai-Szabó, Kriszta Mariann Pencz, Krisztina Ludányi, Nikolett Kállai-Szabó and István Antal
Cited by 5 | Viewed by 2794
Abstract
Today, in addition to many different physicochemical and pharmacological properties of the active ingredients and excipients, the developer of a pharmaceutical formulation must take into account several factors during the formulation process in order for the patient to cooperate to use the formulation
[...] Read more.
Today, in addition to many different physicochemical and pharmacological properties of the active ingredients and excipients, the developer of a pharmaceutical formulation must take into account several factors during the formulation process in order for the patient to cooperate to use the formulation accurately. One of the innovative solutions in paediatrics may be the use of medicated drinking straws. For our studies, we successfully prepared lactase-containing, rapid disintegration particles by two techniques commonly used in the pharmaceutical industry. The simulation of the usage of the filled straws was presented from a new perspective for the patient by an in vitro method. The effect of the temperature of the liquid used during the administration of the straw and the effect of the frequency during the application on the dissolution rate were investigated. According to our results, in the case of a straw containing rapidly dissolving particles, the temperature of the used liquid and the mode of administration (frequency) play a significant role in the release rate from the composition.
Full article
►▼
Show Figures
Open AccessArticle
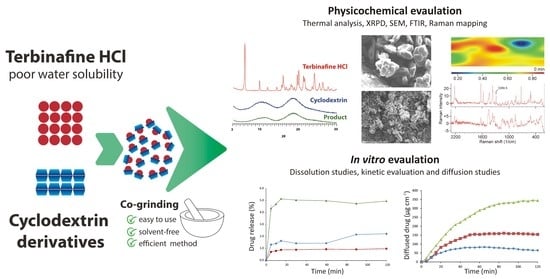
Development of Solvent-Free Co-Ground Method to Produce Terbinafine Hydrochloride Cyclodextrin Binary Systems; Structural and In Vitro Characterizations
by
Balázs Attila Kondoros, Orsolya Jójárt-Laczkovich, Ottó Berkesi, Piroska Szabó-Révész, Ildikó Csóka, Rita Ambrus and Zoltán Aigner
Cited by 6 | Viewed by 2363
Abstract
Molecular complexation with cyclodextrins (CDs) has long been a known process for modifying the physicochemical properties of problematic active pharmaceutical ingredients with poor water solubility. In current times, the focus has been on the solvent-free co-grinding process, which is an industrially feasible process
[...] Read more.
Molecular complexation with cyclodextrins (CDs) has long been a known process for modifying the physicochemical properties of problematic active pharmaceutical ingredients with poor water solubility. In current times, the focus has been on the solvent-free co-grinding process, which is an industrially feasible process qualifying as a green technology. In this study, terbinafine hydrochloride (TER), a low solubility antifungal drug was used as a model drug. This study aimed to prepare co-ground products and follow through the preparation process of the co-grinding method in the case of TER and two amorphous CD derivatives: (2-hydroxypropyl)-β-cyclodextrin (HPBCD); heptakis-(2,6-di-O-methyl)-β-cyclodextrin (DIMEB). For this evaluation, the following analytical tools and methods were used: phase solubility studies, differential scanning calorimetry (DSC), X-ray powder diffractometry (XRPD), hot-stage X-ray powder diffractometry (HOT-XRPD), Fourier-transform infrared (FT-IR), Raman spectroscopy, and Scanning Electron Microscopy (SEM). Furthermore, in vitro characterization (dissolution and diffusion studies) was performed in two kinds of dissolution medium without enzymes. In the XRPD and SEM studies, it was found that the co-grinding of the components resulted in amorphous products. FT-IR and Raman spectroscopies confirmed the formation of an inclusion complex through the unsaturated aliphatic chain of TER and CDs. In vitro characterization suggested better dissolution properties for both CDs and decreased diffusion at higher pH levels in the case of HPBCD.
Full article
►▼
Show Figures
Open AccessArticle
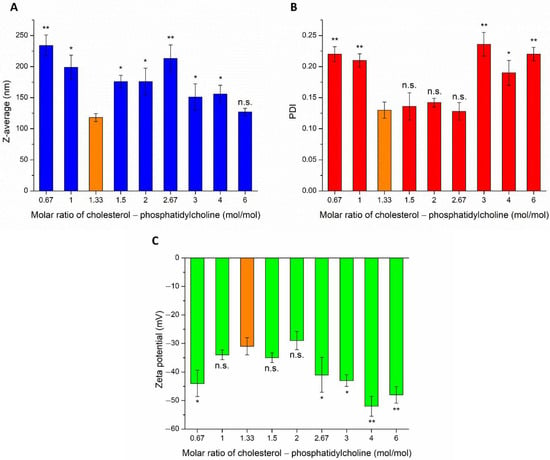
Development of Lomustine and n-Propyl Gallate Co-Encapsulated Liposomes for Targeting Glioblastoma Multiforme via Intranasal Administration
by
Gábor Katona, Fakhara Sabir, Bence Sipos, Muhammad Naveed, Zsuzsanna Schelz, István Zupkó and Ildikó Csóka
Cited by 13 | Viewed by 2726
Abstract
This work aimed to develop lomustine (LOM) and
n-propyl gallate (PG)-loaded liposomes suitable for targeting glioblastoma multiforme (GBM) via the auspicious nose-to-brain drug delivery pathway. The therapeutical effect of LOM, as a nitrosourea compound, can be potentiated by PG suitable for enhanced
[...] Read more.
This work aimed to develop lomustine (LOM) and
n-propyl gallate (PG)-loaded liposomes suitable for targeting glioblastoma multiforme (GBM) via the auspicious nose-to-brain drug delivery pathway. The therapeutical effect of LOM, as a nitrosourea compound, can be potentiated by PG suitable for enhanced anti-cancer therapy. Nose-to-brain delivery of PG and LOM combined in liposomes can overcome the poor water solubility, absorption properties, and toxicity issues in the systemic circulation. Optimization and characterization of the liposomal carrier with binary drug contents were carried out in order to achieve adequate encapsulation efficiency, loading capacity, drug release, and ex vivo permeation. The optimized liposome co-encapsulated with both drugs showed suitable Z-average (127 ± 6.9 nm), size distribution (polydispersity index of 0.142 ± 0.009), zeta potential (−34 ± 1.7 mV), and high encapsulation efficacy (63.57 ± 1.3% of PG and 73.45 ± 2.2% of LOM, respectively) meeting the acceptance criteria of nose-to-brain transport for both drugs. MTT assays of PG-LOM formulations were also conducted on NIH/3T3 (murine embryonic fibroblast), U87 (glioblastoma), and A2780 (ovarian cancer) cell lines indicating reduced an antiproliferative effect on all types of cells. Our results supported the use of this novel combination of LOM and PG in a liposomal formulation as a promising carrier for glioblastoma targeting via the intranasal route.
Full article
►▼
Show Figures
Open AccessArticle
In Vitro and In Vivo Studies of a Verapamil-Containing Gastroretentive Solid Foam Capsule
by
Ádám Haimhoffer, Gábor Vasvári, István Budai, Monika Béresová, Ádám Deák, Norbert Németh, Judit Váradi, Dávid Sinka, Ildikó Bácskay, Miklós Vecsernyés and Ferenc Fenyvesi
Cited by 6 | Viewed by 3851
Abstract
Gastroretentive systems may overcome problems associated with incomplete drug absorption by localized release of the API in the stomach. Low-density drug delivery systems can float in the gastric content and improve the bioavailability of small molecules. The current publication presents verapamil–HCl-containing solid foam
[...] Read more.
Gastroretentive systems may overcome problems associated with incomplete drug absorption by localized release of the API in the stomach. Low-density drug delivery systems can float in the gastric content and improve the bioavailability of small molecules. The current publication presents verapamil–HCl-containing solid foam prepared by continuous manufacturing. Production runs were validated, and the foam structure was characterized by micro-CT scans and SEM. Dissolution properties, texture changes during dissolution, and floating forces were analyzed. An optimized formulation was chosen and given orally to Beagle dogs to determine the pharmacokinetic parameters of the solid foam capsules. As a result, a 12.5 m/m% stearic acid content was found to be the most effective to reduce the apparent density of capsules. Drug release can be described by the first-order model, where 70% of verapamil dissolved after 10 h from the optimized formulation. The texture analysis proved that the structures of the solid foams are resistant. Additionally, the floating forces of the samples remained constant during their dissolution in acidic media. An in vivo study confirmed the prolonged release of the API, and gastroscopic images verified the retention of the capsule in the stomach.
Full article
►▼
Show Figures
Open AccessArticle
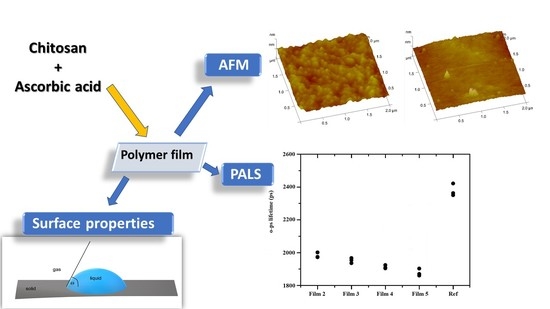
Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid
by
Katalin Kristó, Szilvia Módra, Viktória Hornok, Károly Süvegh, Krisztina Ludasi, Zoltán Aigner, András Kelemen, Tamás Sovány, Klára Pintye-Hódi and Géza Regdon, Jr.
Cited by 9 | Viewed by 2479
Abstract
Nowadays, the buccal administration of mucoadhesive films is very promising. Our aim was to prepare ascorbic acid-containing chitosan films to study the properties and structures important for applicability and optimize the composition. During the formulation of mucoadhesive films, chitosan as the polymer basis
[...] Read more.
Nowadays, the buccal administration of mucoadhesive films is very promising. Our aim was to prepare ascorbic acid-containing chitosan films to study the properties and structures important for applicability and optimize the composition. During the formulation of mucoadhesive films, chitosan as the polymer basis of the film was used. Ascorbic acid, which provided the acidic pH, was used in different concentrations (2–5%). The films were formulated by the solvent casting method. The properties of films important for applicability were investigated, such as physical parameters, mucoadhesive force, surface free energy, and breaking strength. The fine structure of the films was analyzed by atomic force microscopy, and the free volume was analyzed by PALS, which can be important for drug release kinetics and the location of the drug in the film. The applicability of the optimized composition was also tested with two different types of active ingredients. The structure of the films was also analyzed by XRPD and FTIR. Ascorbic acid can be used well in chitosan films, where it can function as a permeation enhancer when reacting to chitosan, it is biodegradable, and can be applied in 2% of our studies.
Full article
►▼
Show Figures
Open AccessArticle
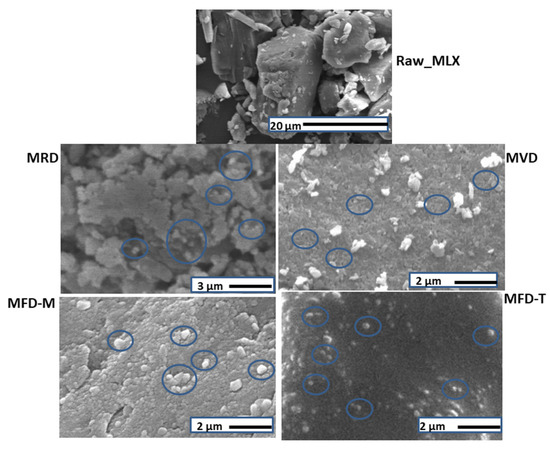
Smartcrystals for Efficient Dissolution of Poorly Water-Soluble Meloxicam
by
Rita Ambrus, Areen Alshweiat, Piroska Szabó-Révész, Csilla Bartos and Ildikó Csóka
Cited by 11 | Viewed by 2235
Abstract
Nanocrystal is widely applied to improve the dissolution of poorly water-soluble drugs. We aimed to prepare meloxicam (MLX) nanocrystals using the bead mill method, followed by high-pressure homogenization (HPH). Simple drying at room temperature (RD), vacuum-drying (VD), and freeze-drying (FD) using mannitol or
[...] Read more.
Nanocrystal is widely applied to improve the dissolution of poorly water-soluble drugs. We aimed to prepare meloxicam (MLX) nanocrystals using the bead mill method, followed by high-pressure homogenization (HPH). Simple drying at room temperature (RD), vacuum-drying (VD), and freeze-drying (FD) using mannitol or trehalose as a cryoprotectant were applied to obtain dry nanocrystals. The nanocrystals were fully characterized. The MLX nanosuspension containing 5%
w/v MLX and 1%
w/v of Pluronic F68 showing a mean particle size (MPS) of 242 nm and a polydispersity index (PDI) of 0.36 was prepared after 40 min of premilling and 30 min of HPH. The dried nanocrystals were spherical within the nano range. DSC and XRPD confirmed the absence of MLX amorphization. The smartcrystals showed enhanced MLX release. Approximately 100% release was achieved with phosphate buffer (PB), pH 5.6, and 80% was released with PB, pH 7.4, from the freeze-dried samples. The results revealed the effects of the drying method and cryoprotectant type on the properties of dry nanocrystals. The freeze-dried samples showed the smallest particle size, in particular trehalose-based samples. On the other hand, mannitol-based dried samples showed the highest crystallinity index among all nanocrystals (77.8%), whereas trehalose showed the lowest (59.2%). These factors explained the dissolution differences among the samples.
Full article
►▼
Show Figures
Open AccessArticle
Predicting Drug Release Rate of Implantable Matrices and Better Understanding of the Underlying Mechanisms through Experimental Design and Artificial Neural Network-Based Modelling
by
Ernő Benkő, Ilija German Ilič, Katalin Kristó, Géza Regdon, Jr., Ildikó Csóka, Klára Pintye-Hódi, Stane Srčič and Tamás Sovány
Cited by 2 | Viewed by 2003
Abstract
There is a growing interest in implantable drug delivery systems (DDS) in pharmaceutical science. The aim of the present study is to investigate whether it is possible to customize drug release from implantable DDSs through drug–carrier interactions. Therefore, a series of chemically similar
[...] Read more.
There is a growing interest in implantable drug delivery systems (DDS) in pharmaceutical science. The aim of the present study is to investigate whether it is possible to customize drug release from implantable DDSs through drug–carrier interactions. Therefore, a series of chemically similar active ingredients (APIs) was mixed with different matrix-forming materials and was then compressed directly. Compression and dissolution interactions were examined by FT-IR spectroscopy. Regarding the effect of the interactions on drug release kinetics, a custom-made dissolution device designed for implantable systems was used. The data obtained were used to construct models based on artificial neural networks (ANNs) to predict drug dissolution. FT-IR studies confirmed the presence of H-bond-based solid-state interactions that intensified during dissolution. These results confirmed our hypothesis that interactions could significantly affect both the release rate and the amount of the released drug. The efficiencies of the kinetic parameter-based and point-to-point ANN models were also compared, where the results showed that the point-to-point models better handled predictive inaccuracies and provided better overall predictive efficiency.
Full article
►▼
Show Figures
Open AccessArticle
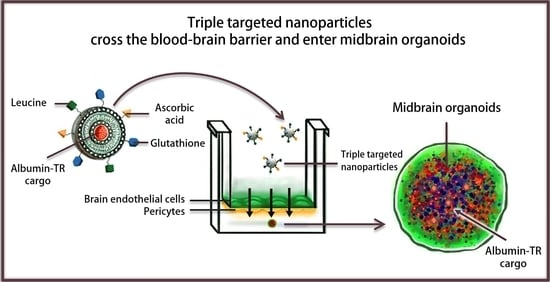
A Triple Combination of Targeting Ligands Increases the Penetration of Nanoparticles across a Blood-Brain Barrier Culture Model
by
Szilvia Veszelka, Mária Mészáros, Gergő Porkoláb, Anikó Szecskó, Nóra Kondor, Györgyi Ferenc, Tamás F. Polgár, Gábor Katona, Zoltán Kóta, Lóránd Kelemen, Tibor Páli, Judit P. Vigh, Fruzsina R. Walter, Silvia Bolognin, Jens C. Schwamborn, Jeng-Shiung Jan and Mária A. Deli
Cited by 8 | Viewed by 3490
Abstract
Nanosized drug delivery systems targeting transporters of the blood-brain barrier (BBB) are promising carriers to enhance the penetration of therapeutics into the brain. The expression of solute carriers (SLC) is high and shows a specific pattern at the BBB. Here we show that
[...] Read more.
Nanosized drug delivery systems targeting transporters of the blood-brain barrier (BBB) are promising carriers to enhance the penetration of therapeutics into the brain. The expression of solute carriers (SLC) is high and shows a specific pattern at the BBB. Here we show that targeting ligands ascorbic acid, leucine and glutathione on nanoparticles elevated the uptake of albumin cargo in cultured primary rat brain endothelial cells. Moreover, we demonstrated the ability of the triple-targeted nanovesicles to deliver their cargo into midbrain organoids after crossing the BBB model. The cellular uptake was temperature- and energy-dependent based on metabolic inhibition. The process was decreased by filipin and cytochalasin D, indicating that the cellular uptake of nanoparticles was partially mediated by endocytosis. The uptake of the cargo encapsulated in triple-targeted nanoparticles increased after modification of the negative zeta potential of endothelial cells by treatment with a cationic lipid or after cleaving the glycocalyx with an enzyme. We revealed that targeted nanoparticles elevated plasma membrane fluidity, indicating the fusion of nanovesicles with endothelial cell membranes. Our data indicate that labeling nanoparticles with three different ligands of multiple transporters of brain endothelial cells can promote the transfer and delivery of molecules across the BBB.
Full article
►▼
Show Figures
Open AccessArticle
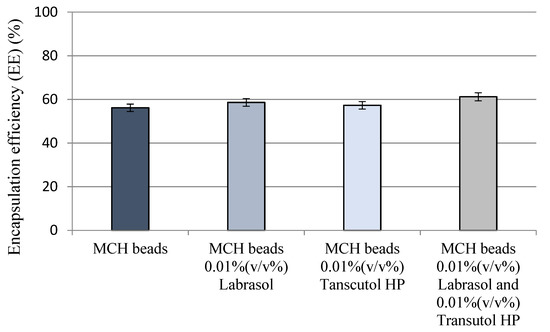
Oral Bioavailability Enhancement of Melanin Concentrating Hormone, Development and In Vitro Pharmaceutical Assessment of Novel Delivery Systems
by
Dóra Kósa, Ágota Pető, Ferenc Fenyvesi, Judit Váradi, Miklós Vecsernyés, István Budai, József Németh, Pálma Fehér, Ildikó Bácskay and Zoltán Ujhelyi
Cited by 3 | Viewed by 2883
Abstract
The rapid progress in biotechnology over the past few decades has accelerated the large-scale production of therapeutic peptides and proteins, making them available in medical practice. However, injections are the most common method of administration; these procedures might lead to inconvenience. Non-invasive medications,
[...] Read more.
The rapid progress in biotechnology over the past few decades has accelerated the large-scale production of therapeutic peptides and proteins, making them available in medical practice. However, injections are the most common method of administration; these procedures might lead to inconvenience. Non-invasive medications, such as oral administration of bio-compounds, can reduce or eliminate pain and increase safety. The aim of this project was to develop and characterize novel melanin concentrating hormone (MCH) formulations for oral administration. As a drug delivery system, penetration enhancer combined alginate beads were formulated and characterized. The combination of alginate carriers with amphiphilic surfactants has not been described yet. Due to biosafety having high priority in the case of novel pharmaceutical formulations, the biocompatibility of selected auxiliary materials and their combinations was evaluated using different in vitro methods. Excipients were selected according to the performed toxicity measurements. Besides the cell viability tests, physical properties and complex bioavailability assessments were performed as well. Our results suggest that alginate beads are able to protect melanin concentrating hormones. It has been also demonstrated that penetration enhancer combined alginate beads might play a key role in bioavailability improvement. These formulations were found to be promising tools for oral peptide delivery. Applied excipients and the performed delivery systems are safe and highly tolerable; thus, they can improve patients’ experience and promote adherence.
Full article
►▼
Show Figures
Open AccessArticle
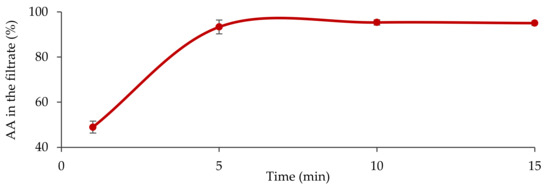
Dose Finding and Food Effect Studies of a Novel Abiraterone Acetate Formulation for Oral Suspension in Comparison to a Reference Formulation in Healthy Male Subjects
by
Tamás Jordán, Orsolya Basa-Dénes, Réka Angi, János Orosz, Zsolt Ötvös, Andrea Ujhelyi, Genovéva Filipcsei, László Molnár, Tamás Solymosi, Hristos Glavinas, Dominic Capone, Nicola Whitfield, John McDermott, Litza McKenzie, Lauren Shurety and Elizabeth Manning Duus
Cited by 2 | Viewed by 2372
Abstract
Currently approved formulations of the androgen synthesis inhibitor abiraterone acetate (AA) consist of multiple tablets administered daily in a fasted state. Removing the food effect and switching to a suspension formulation is expected to improve the pharmacokinetic profile and facilitate drug administration for
[...] Read more.
Currently approved formulations of the androgen synthesis inhibitor abiraterone acetate (AA) consist of multiple tablets administered daily in a fasted state. Removing the food effect and switching to a suspension formulation is expected to improve the pharmacokinetic profile and facilitate drug administration for patients with late-stage prostate cancer. Two four-sequence, four-period randomized crossover investigations were undertaken to establish the pharmacokinetic profiles of single doses of commercially available Zytiga
®, as the reference AA (R-AA), and a novel tablet for oral suspension (TOS). Four single doses of TOS (from 62.5 to 250 mg) were compared in study C01, and two single doses each of TOS (250 mg) and R-AA (1000 mg) were compared under fasted and fed (modified fasted for R-AA) conditions in C02. Plasma concentrations of abiraterone over time were measured, and pharmacokinetic parameters were calculated. Each doubling of the dose of TOS was associated with a greater than 3-fold increase in exposure. A single dose of TOS (250 mg) exhibited similar exposure over 24 h, whether given fasted (625 ng × h/mL) or fed (485 ng × h/mL). A single dose of TOS (250 mg) was associated with higher (fasted,
p = 0.028) or equivalent exposure (fed) compared to 1000 mg R-AA fasted (532 ng × h/mL). Substantially higher exposures were seen with 1000 mg R-AA under modified fasted conditions compared to TOS, irrespective of prandial status (
p < 0.001). TOS was generally safe and well tolerated in the study. A 250 mg dose of a novel AA formulation for oral suspension demonstrated bioequivalence to 1000 mg R-AA under fasted conditions. This novel TOS formulation also addresses some of the limitations of current AA treatment, including low bioavailability, high variability in systemic exposure and a large food effect. It may offer an alternative for patients with dysphagia or discomfort with swallowing large pills.
Full article
►▼
Show Figures
Open AccessArticle
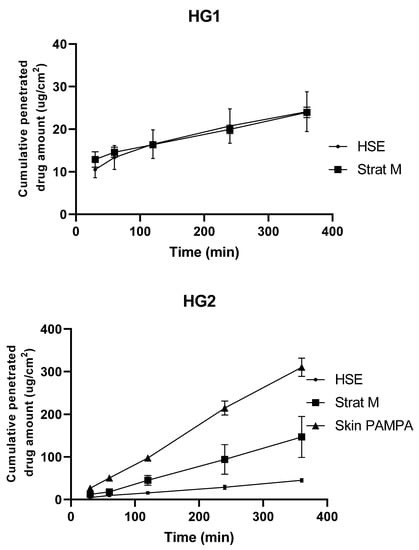
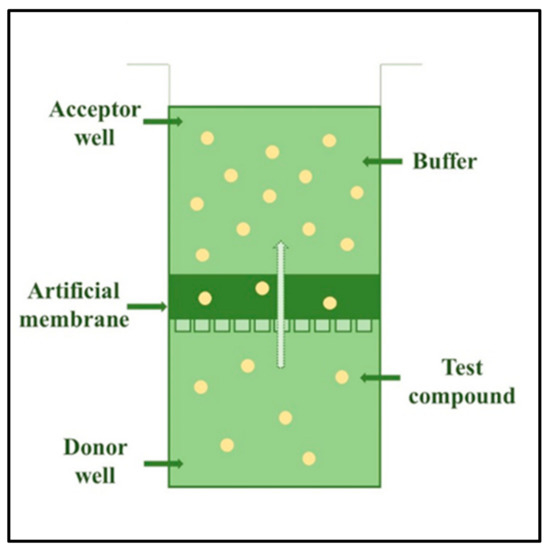
Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro
by
Anita Kovács, Stella Zsikó, Fanni Falusi, Erzsébet Csányi, Mária Budai-Szűcs, Ildikó Csóka and Szilvia Berkó
Cited by 11 | Viewed by 2822
Abstract
In recent years, the study of dermal preparations has received increased attention. There are more and more modern approaches to evaluate transdermal formulations, which are crucial in proving the efficacy of a formulation. The aim of this study was to compare permeation across
[...] Read more.
In recent years, the study of dermal preparations has received increased attention. There are more and more modern approaches to evaluate transdermal formulations, which are crucial in proving the efficacy of a formulation. The aim of this study was to compare permeation across innovative synthetic membranes (Strat-M and Skin PAMPA membranes) and heat-separated human epidermis (HSE, gold standard membrane) using four different dermal formulations. The Strat-M and Skin PAMPA membranes were designed to mimic the stratum corneum layer of the human epidermis. There have also been some publications on their use in dermal formulation development, but further information is needed. Drug permeation was measured using formulations containing diclofenac sodium (two hydrogels and two creams). The HSE, Strat-M, and Skin PAMPA membranes proved to be significantly different, but based on the results, the Strat-M membrane showed the greatest similarity to HSE. The permeation data of the different formulations across different membranes showed good correlations with formulations similar to these four, which allows the prediction of permeation across HSE using these synthetic membranes. In addition, Strat-M and Skin PAMPA membranes have the potential to select and differentiate a dermal formulation containing diclofenac sodium as an early screening model.
Full article
►▼
Show Figures
Open AccessArticle
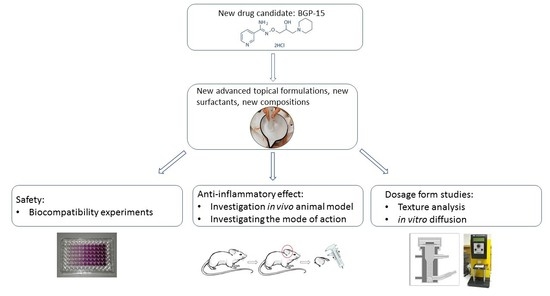
Nicotinic Amidoxime Derivate BGP-15, Topical Dosage Formulation and Anti-Inflammatory Effect
by
Ágota Pető, Dóra Kósa, Ádám Haimhoffer, Pálma Fehér, Zoltán Ujhelyi, Dávid Sinka, Ferenc Fenyvesi, Judit Váradi, Miklós Vecsernyés, Alexandra Gyöngyösi, István Lekli, Péter Szentesi, Annamária Marton, Imre Gombos, Barbara Dukic, László Vígh and Ildikó Bácskay
Cited by 5 | Viewed by 2217
Abstract
BGP-15 is a Hungarian-developed drug candidate with numerous beneficial effects. Its potential anti-inflammatory effect is a common assumption, but it has not been investigated in topical formulations yet. The aim of our study was to formulate 10% BGP-15 creams with different penetration enhancers
[...] Read more.
BGP-15 is a Hungarian-developed drug candidate with numerous beneficial effects. Its potential anti-inflammatory effect is a common assumption, but it has not been investigated in topical formulations yet. The aim of our study was to formulate 10% BGP-15 creams with different penetration enhancers to ensure good drug delivery, improve bioavailability of the drug and investigate the potential anti-inflammatory effect of BGP-15 creams in vivo. Since the exact mechanism of the effect is still unknown, the antioxidant effect (tested with UVB radiation) and the ability of BGP-15 to decrease macrophage activation were evaluated. Biocompatibility investigations were carried out on HaCaT cells to make sure that the formulations and the selected excipients can be safely used. Dosage form studies were also completed with texture analysis and in vitro release with Franz diffusion chamber apparatus. Our results show that the ointments were able to reduce the extent of local inflammation in mice, but the exact mechanism of the effect remains unknown since BGP-15 did not show any antioxidant effect, nor was it able to decrease LPS-induced macrophage activation. Our results support the hypothesis that BGP-15 has a potential anti-inflammatory effect, even if it is topically applied, but the mechanism of the effect remains unclear and requires further pharmacological studies.
Full article
►▼
Show Figures
Open AccessReview
Skin-on-a-Chip Technology for Testing Transdermal Drug Delivery—Starting Points and Recent Developments
by
Zsófia Varga-Medveczky, Dorottya Kocsis, Márton Bese Naszlady, Katalin Fónagy and Franciska Erdő
Cited by 18 | Viewed by 3699
Abstract
During the last decades, several technologies were developed for testing drug delivery through the dermal barrier. Investigation of drug penetration across the skin can be important in topical pharmaceutical formulations and also in cosmeto-science. The state-of- the-art in the field of skin diffusion
[...] Read more.
During the last decades, several technologies were developed for testing drug delivery through the dermal barrier. Investigation of drug penetration across the skin can be important in topical pharmaceutical formulations and also in cosmeto-science. The state-of- the-art in the field of skin diffusion measurements, different devices, and diffusion platforms used, are summarized in the introductory part of this review. Then the methodologies applied at Pázmány Péter Catholic University are shown in detail. The main testing platforms (Franz diffusion cells, skin-on-a-chip devices) and the major scientific projects (P-glycoprotein interaction in the skin; new skin equivalents for diffusion purposes) are also presented in one section. The main achievements of our research are briefly summarized: (1) new skin-on-a-chip microfluidic devices were validated as tools for drug penetration studies for the skin; (2) P-glycoprotein transport has an absorptive orientation in the skin; (3) skin samples cannot be used for transporter interaction studies after freezing and thawing; (4) penetration of hydrophilic model drugs is lower in aged than in young skin; (5) mechanical sensitization is needed for excised rodent and pig skins for drug absorption measurements. Our validated skin-on-a-chip platform is available for other research groups to use for testing and for utilizing it for different purposes.
Full article
►▼
Show Figures
Open AccessArticle
Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing
by
Petra Arany, Ildikó Papp, Marianna Zichar, Géza Regdon, Jr., Mónika Béres, Melinda Szalóki, Renátó Kovács, Pálma Fehér, Zoltán Ujhelyi, Miklós Vecsernyés and Ildikó Bácskay
Cited by 21 | Viewed by 4734
Abstract
Vaginal drug delivery systems can provide a long-term and constant liberation of the active pharmaceutical ingredient even for months. For our experiment, FDM 3D printing was used to manufacture the vaginal ring samples from thermoplastic polyurethane filament, which enables fast manufacturing of complex,
[...] Read more.
Vaginal drug delivery systems can provide a long-term and constant liberation of the active pharmaceutical ingredient even for months. For our experiment, FDM 3D printing was used to manufacture the vaginal ring samples from thermoplastic polyurethane filament, which enables fast manufacturing of complex, personalized medications. 3D printing can be an excellent alternative instead of industrial manufacturing, which is complicated and time-consuming. In our work, the 3D printed vaginal rings were filled manually with jellified metronidazole or chloramphenicol for the treatment of bacterial vaginosis. The need for manual filling was certified by the thermogravimetric and heatflow assay results. The manufactured samples were analyzed by an Erweka USP type II Dissolution Apparatus, and the dissolution profile can be distinguished based on the applied jellifying agents and the API’s. All samples were considered non-similar based on the pairwise comparison. The biocompatibility properties were determined by prolonged MTT assay on HeLa cells, and the polymer could be considered non-toxic. Based on the microbiological assay on
E. coli metronidazole and chitosan containing samples had bactericidal effects while just metronidazole or just chitosan containing samples bacteriostatic effect. None of these samples showed a fungistatic or fungicide effect against
C. albicans. Based on our results, we successfully manufactured 3D printed vaginal rings filled with jellified metronidazole.
Full article
►▼
Show Figures
Open AccessReview
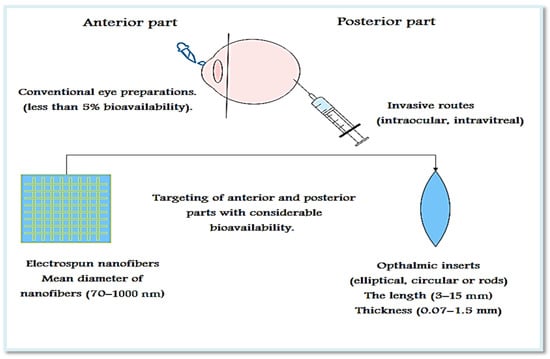
A Systematic Review of Drug-Loaded Electrospun Nanofiber-Based Ophthalmic Inserts
by
Safaa Omer and Romána Zelkó
Cited by 18 | Viewed by 3410
Abstract
Currently, ocular inserts and nanoparticles have received much attention due to the limited bioavailability of conventional eye preparations and the toxicity problems of systemic drug administration. The current systematic review aims to present recent studies on the use of electrospun nanofiber-based ocular inserts
[...] Read more.
Currently, ocular inserts and nanoparticles have received much attention due to the limited bioavailability of conventional eye preparations and the toxicity problems of systemic drug administration. The current systematic review aims to present recent studies on the use of electrospun nanofiber-based ocular inserts to improve the bioavailability of drugs used for different ophthalmic diseases. A systematic search was performed in PubMed, Ovid Medline, Web of Science, ScienceDirect, Scopus, Reaxys, Google Scholar, and Google Patents/Espacenet taking “drug-loaded”, “nanofibers”, and “ophthalmic inserts” and their equivalent terms as keywords. The search was limited to original and peer-reviewed studies published in 2011–2021 in English language. Only 13 out of 795 articles and 15 out of 197 patents were included. All results revealed the success of nanofiber-based ocular inserts in targeting and improved bioavailability. Ocular inserts based on nanofibers can be used as safe, efficient carriers for the treatment of anterior and posterior eye diseases.
Full article
►▼
Show Figures