Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts
Abstract
:1. Introduction
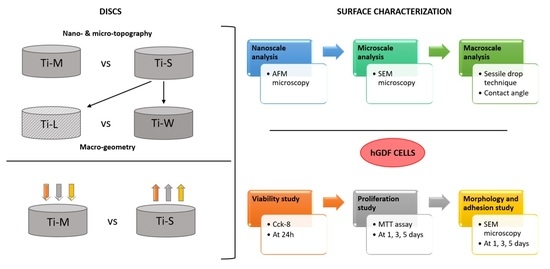
2. Results
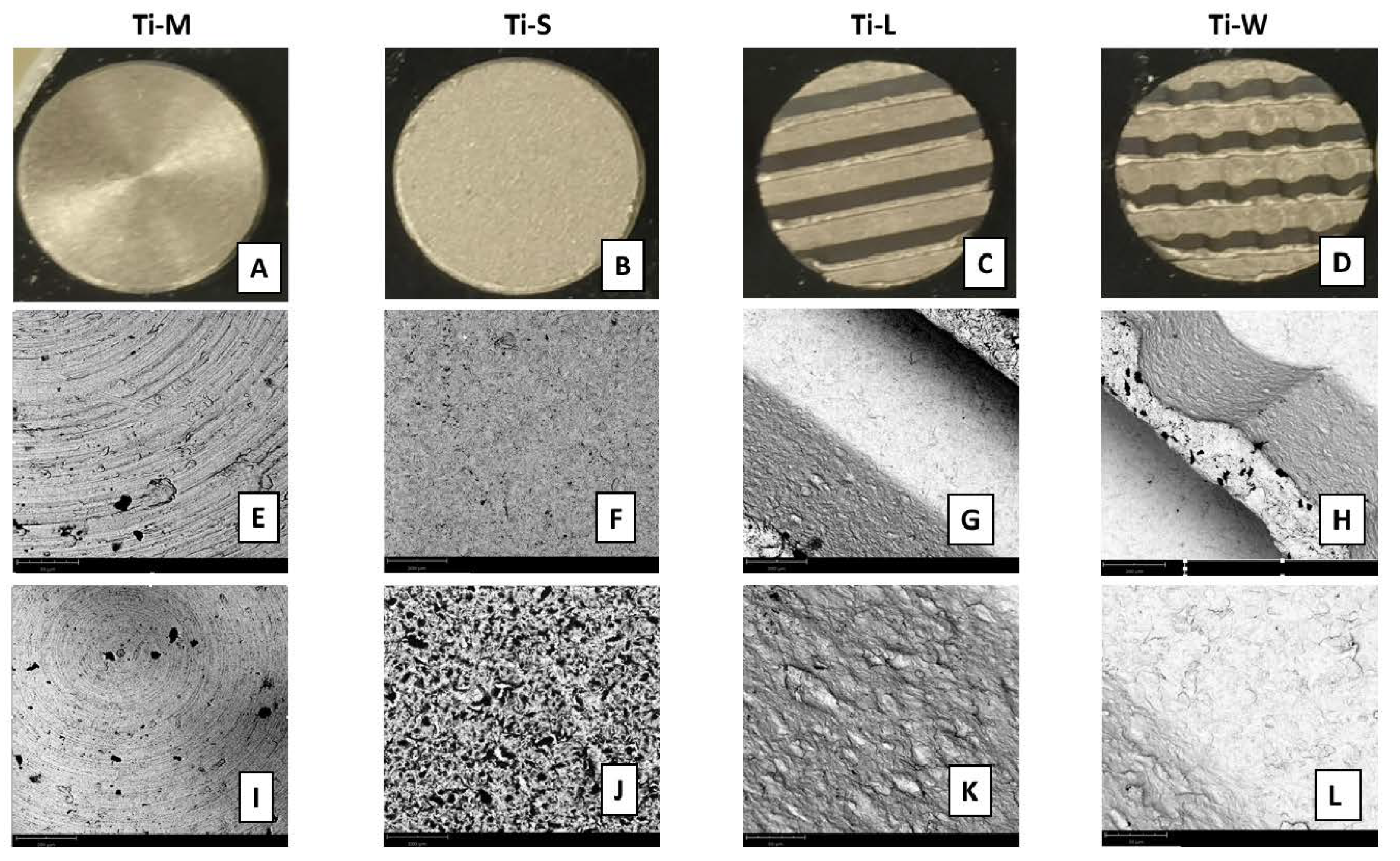
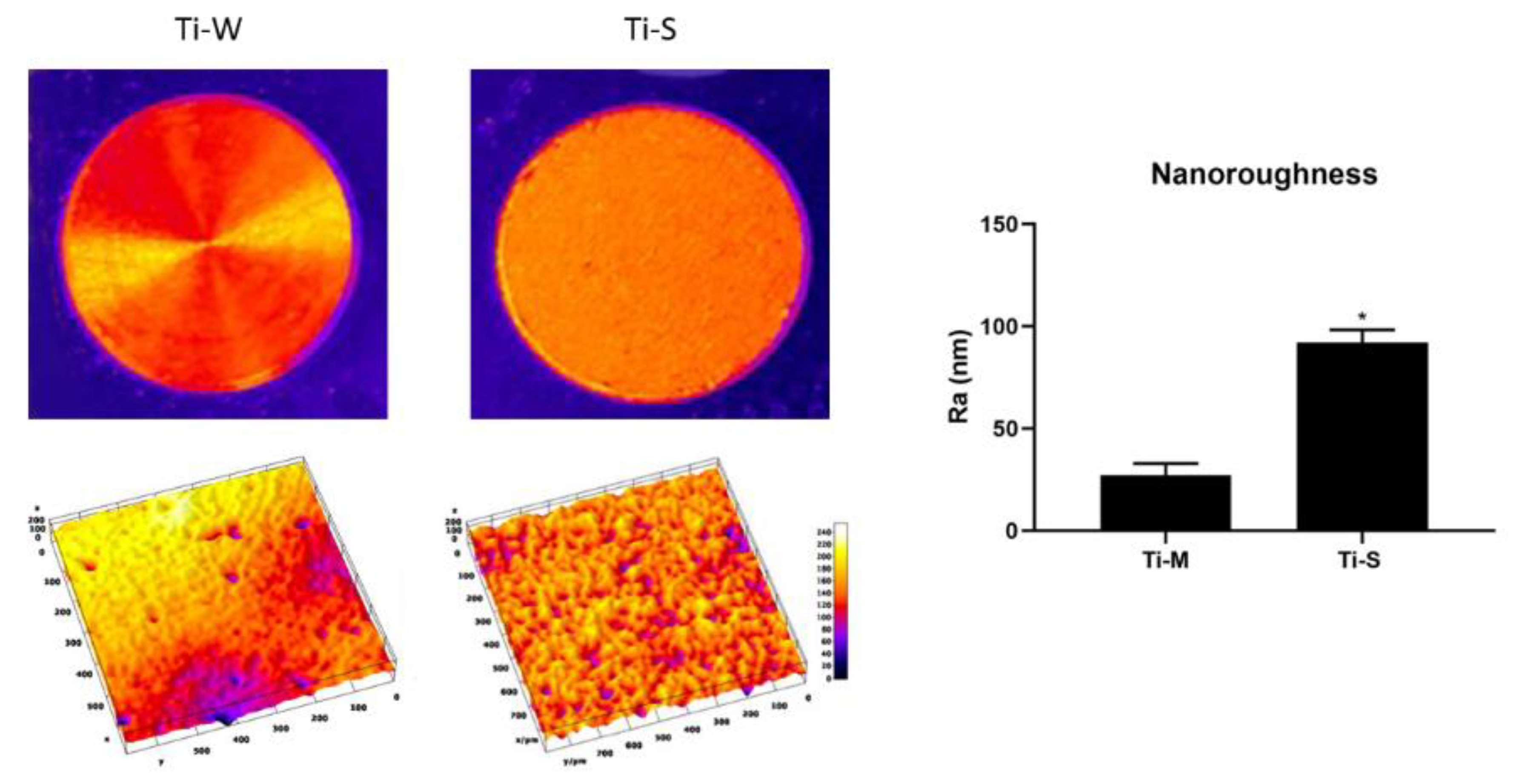
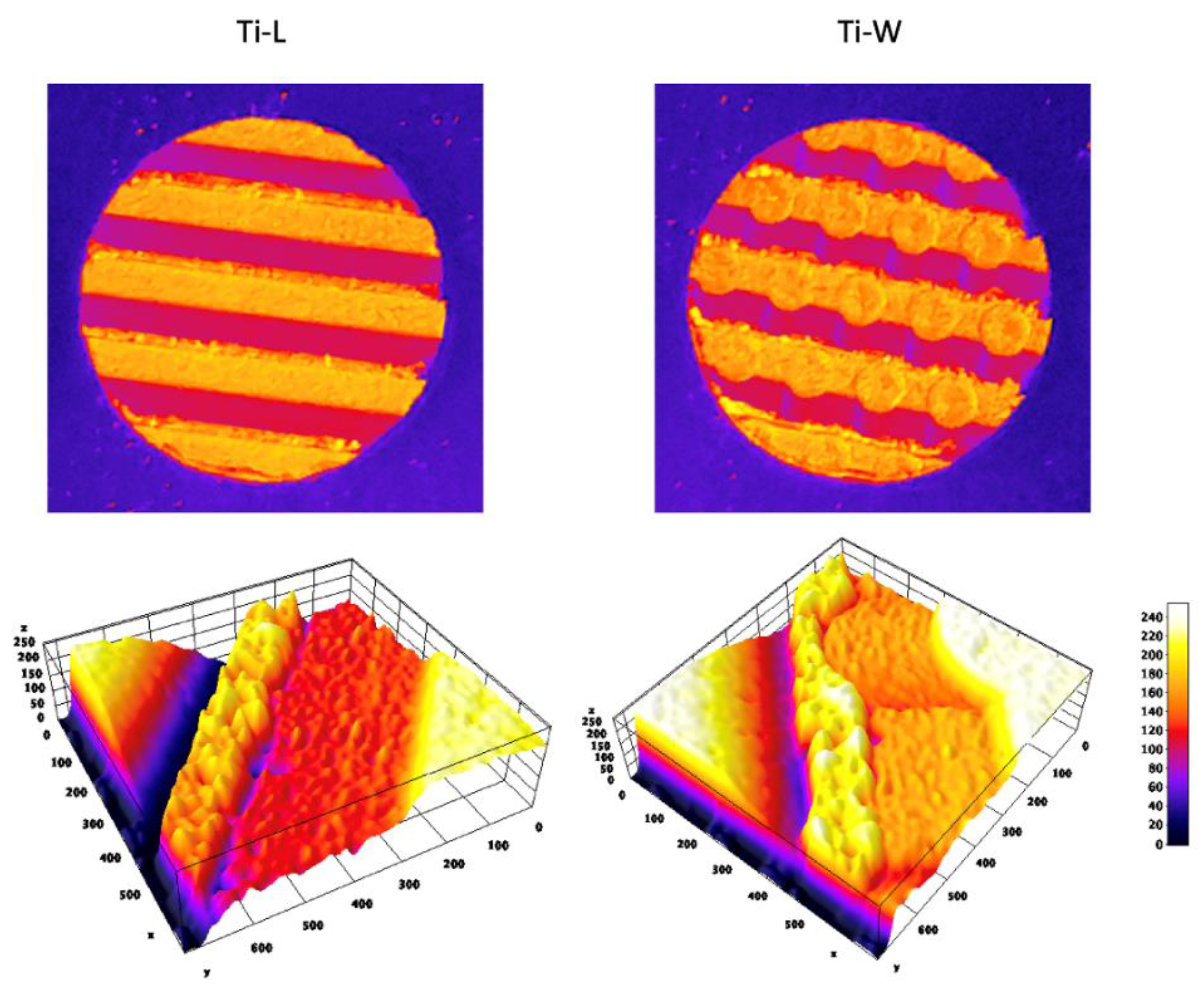
2.1. Surface Topography
2.2. Cell Morphology and Adhesion
2.3. Cell Viability
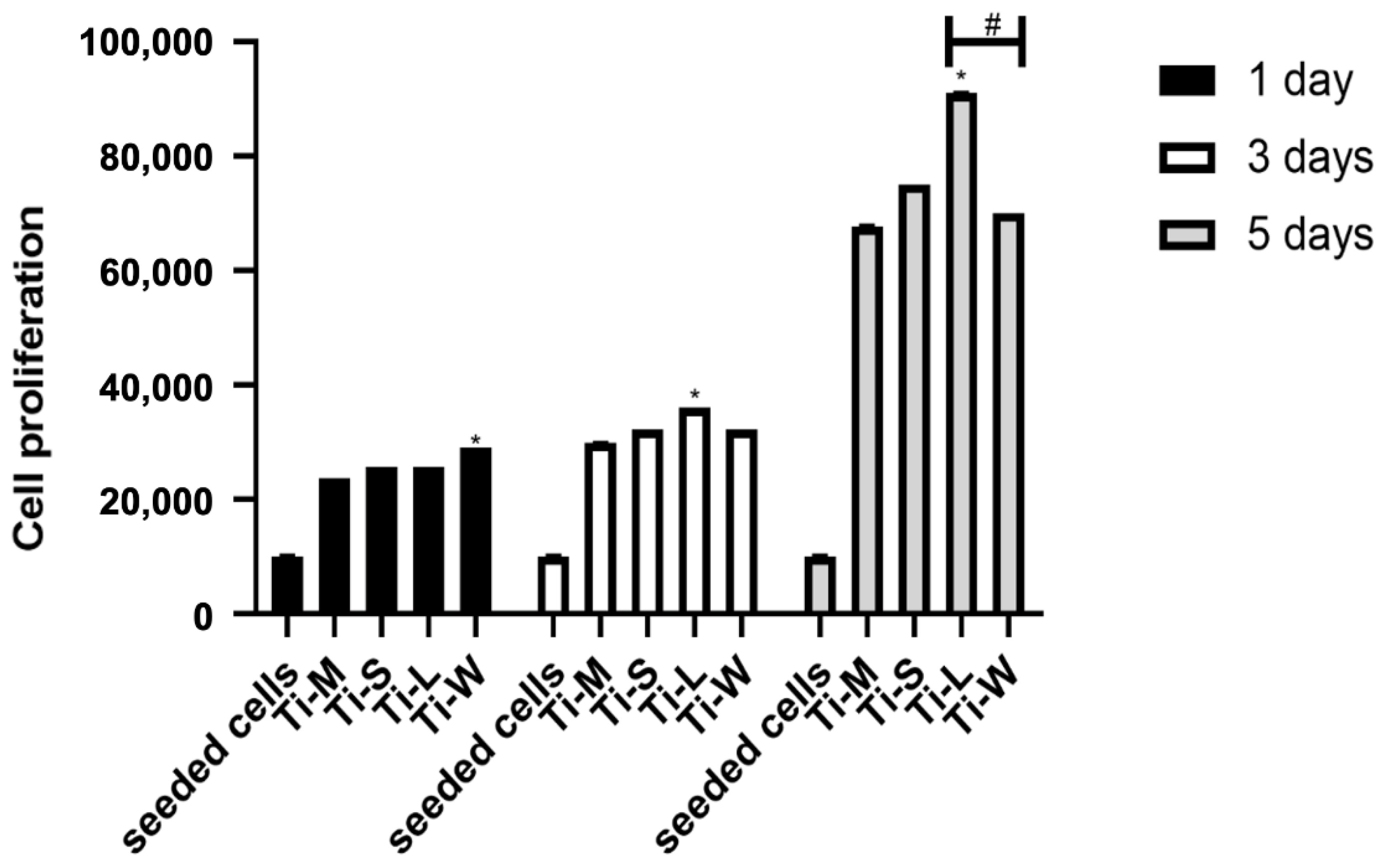
2.4. Cell Proliferation
3. Discussion
4. Material and Methods
4.1. Dental Implant
4.2. Surface Characterization
4.2.1. AFM Analysis for Nanoscale Characterization
4.2.2. SEM Analysis for Microscale Characterization
4.2.3. Wettability Analysis for Macroscale Characterization
4.3. Biological Analysis
4.3.1. Cell Culture
4.3.2. Evaluation of Morphology and Adhesion
4.3.3. Cell Viability Assay
4.3.4. Cell Proliferation Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Elani, H.; Starr, J.; Da Silva, J.; Gallucci, G. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Perrotti, V.; Piattelli, A.; Piemontese, M.; Procaccini, M. Procaccini, Implants placed in sites of previously failed implants: A systematic review, Implant. Dent. 2014, 23, 311–318. [Google Scholar] [CrossRef]
- Grusovin, M.G.; Kakisis, I.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of perimplantitis. Cochrane Database Syst. Rev. 2008, 52, CD004970. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [Green Version]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Assenza, B.; Tripodi, D.; Scarano, A.; Perrotti, V.; Piattelli, A.; Iezzi, G.; D'Ercole, S. Bacterial Leakage in Implants With Different Implant–Abutment Connections: An In Vitro Study. J. Periodontol. 2012, 83, 491–497. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Piattelli, A.; Scarano, A.; Shibli, J.A.; Piccirilli, M.; Perrotti, V.; Iezzi, G. Histological and immunohistochemical evaluation of the peri-implant soft tissues around machined and acid-etched titanium healing abutments: A prospective randomised study. Clin. Oral Investig. 2011, 16, 857–866. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Nobles, K.P.; Janorkar, A.V.; Williamson, R.S. Surface modifications to enhance osseointegration–Resulting material properties and biological responses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 1, 1–15. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The Bacterial Anti-Adhesive Activity of Double-Etched Titanium (DAE) as a Dental Implant Surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Glantz, P.-O.; Lindhe, J. The mucosal attachment at different abutments. An experimental study in dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Júnior, J.A.; Pérez-Díaz, L.; Treichel, T.L.E.; DeDavid, B.A.; De Aza, P.N.; Frutos, J.C.P. New Implant Macrogeometry to Improve and Accelerate the Osseointegration: An In Vivo Experimental Study. Appl. Sci. 2019, 9, 3181. [Google Scholar] [CrossRef] [Green Version]
- Gehrke, S.A.; Tumedei, M.; Júnior, J.A.; Treichel, T.L.E.; Kolerman, R.; Lepore, S.; Piattelli, A.; Iezzi, G. Histological and Histomorphometrical Evaluation of a New Implant Macrogeometry. A Sheep Study. Int. J. Environ. Res. Public Health 2020, 17, 3477. [Google Scholar] [CrossRef]
- Shibli, J.A.; Pires, J.T.; Piattelli, A.; Iezzi, G.; Mangano, C.; Mangano, F.; De Souza, S.L.; Gehrke, S.A.; Wang, H.-L.; Ehrenfest, D.M.D. Impact of Different Implant Surfaces Topographies on Peri-Implant Tissues: An Update of Current Available Data on Dental Implants Retrieved from Human Jaws. Curr. Pharm. Biotechnol. 2017, 18, 76–84. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the periimplant mucosa Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A 30-Year (1988–2018) Retrospective Microscopical Evaluation of Dental Implants Retrieved for Different Causes: A Narrative Review. Int. J. Periodontics Restor. Dent. 2020, 40, e211–e227. [Google Scholar] [CrossRef]
- Schmidt, K.E.; Auschill, T.M.; Heumann, C.; Frankenberger, R.; Eick, S.; Sculean, A.; Arweiler, N.B. Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin. Oral Implant. Res. 2016, 28, 483–490. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- D’Ercole, S.; Scarano, A.; Perrotti, V.; Mulatinho, J.; Piattelli, A.; Iezzi, G.; Tripodi, D. Implants with Internal Hexagon and Conical Implant-Abutment Connections: An In Vitro Study of the Bacterial Contamination. J. Oral Implant. 2014, 40, 30–34. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, A.; Polimeni, A.; Di Iorio, D.; Carinci, F. Bacterial Adhesion on Commercially Pure Titanium and Anatase-Coated Titanium Healing Screws: An In Vivo Human Study. J. Periodontol. 2010, 81, 1466–1471. [Google Scholar] [CrossRef]
- D’Ercole, S.; di Campli, E.; Pilato, S.; Iezzi, G.; Cellini, L.; Piattelli, A.; Petrini, M. Streptococcus oralis biofilm formation on titanium surfaces. Topographical characteristics and bacterial interaction with different titanium surfaces. Int. J. Oral Maxillofac. Implant. 2021, accepted. [Google Scholar]
- Diomede, F.; Caputi, S.; Merciaro, I.; Frisone, S.; D’Arcangelo, C.; Piattelli, A.; Trubiani, O. Pro-inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. Int. Endod. J. 2014, 47, 864–872. [Google Scholar] [CrossRef]
- Ferraris, S.; Bobbio, A.; Miola, M.; Spriano, S. Micro- and nano-textured, hydrophilic and bioactive titanium dental implants. Surf. Coatings Technol. 2015, 276, 374–383. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2012, 9, 6268–6277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comuzzi, L.; Tumedei, M.; De Angelis, F.; Lorusso, F.; Piattelli, A.; Iezzi, G. Influence of the dental implant macrogeometry and threads design on primary stability: An in vitro simulation on artificial bone blocks. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1242–1250. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Aramburú, J.; Pérez-Díaz, L.; Prado, T.D.D.; DeDavid, B.A.; Mazon, P.; De Aza, P.N. Can changes in implant macrogeometry accelerate the osseointegration process?: An in vivo experimental biomechanical and histological evaluations. PLoS ONE 2020, 15, e0233304. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Gennai, S.; Petrini, M.; Bettini, L.; Tonetti, M. Enamel matrix derivative stabilizes blood clot and improves clinical healing in deep pockets after flapless periodontal therapy: A Randomized Clinical Trial. J. Clin. Periodontol. 2019, 46, 231–240. [Google Scholar] [CrossRef]




| Antigenes | Expression Levels |
|---|---|
| CD 73 | + |
| CD 90 | + |
| CD 105 | + |
| CD 45 | − |
| CD 326 | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Di Pietro, N.; Pandolfi, A.; D’Arcangelo, C.; De Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A.; et al. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189871
Petrini M, Pierfelice TV, D’Amico E, Di Pietro N, Pandolfi A, D’Arcangelo C, De Angelis F, Mandatori D, Schiavone V, Piattelli A, et al. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. International Journal of Molecular Sciences. 2021; 22(18):9871. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189871
Chicago/Turabian StylePetrini, Morena, Tania Vanessa Pierfelice, Emira D’Amico, Natalia Di Pietro, Assunta Pandolfi, Camillo D’Arcangelo, Francesco De Angelis, Domitilla Mandatori, Valeria Schiavone, Adriano Piattelli, and et al. 2021. "Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts" International Journal of Molecular Sciences 22, no. 18: 9871. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189871