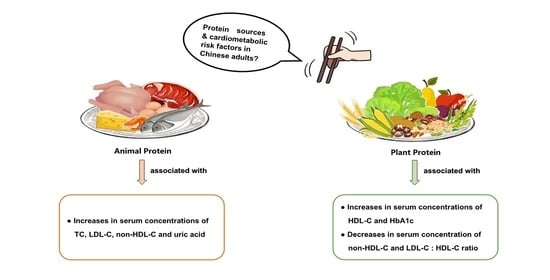
Associations between Dietary Animal and Plant Protein Intake and Cardiometabolic Risk Factors—A Cross-Sectional Study in China Health and Nutrition Survey
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Dietary Assessment
2.3. Assessment of CMRF
2.4. Assessment of Other Covariates
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic, Anthropometric and Lifestyle Characteristics of Participants
3.2. Food Sources of Total, Animal, and Plant Protein
3.3. Associations Between Dietary Total, Animal, and Plant Protein Intakes and CMRFs
3.4. Subgroup Analyses on the Basis of BMI, Sex, Age, and Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | branched-chain amino acid |
| BMI | body mass index |
| CHNS | China Health and Nutrition Survey |
| CMRF | cardiometabolic risk factor |
| DBP | diastolic blood pressure |
| EAAs | essential amino acids |
| HbA1c | hemoglobin A1c |
| HDL-C | high-density lipoprotein cholesterol |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| hsCRP | high-sensitive C-reactive protein |
| LDL-C | low-density lipoprotein cholesterol |
| MUFA | monounsaturated fatty acids |
| NEAAs | nonessential amino acids |
| PUFA | polyunsaturated fatty acids |
| RCT | randomized, double-blind controlled trial |
| SBP | systolic blood pressure |
| SFA | saturated fatty acids |
| TMAO | trimethylamine-N-oxide |
| TC | total cholesterol |
| TG | triglycerides |
References
- WHO. The Top Ten Causes of Death. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 May 2020).
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation (IDF). IDF DIABETES ATLAS 9th edition 2019. Available online: https://diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html (accessed on 11 November 2020).
- Richter, C.; Skulas-Ray, A.; Kris-Etherton, P. Chapter 27—The Role of Diet in the Prevention and Treatment of Cardio-vascular Disease. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Delahanty, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 595–623. [Google Scholar] [CrossRef]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: ADose-Response Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-Carbohydrate-Diet Score and the Risk of Coronary Heart Disease in Women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major Dietary Protein Sources and Risk of Coronary Heart Disease in Women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Preis, S.R.; Stampfer, M.J.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Dietary protein and risk of ischemic heart disease in middle-aged men. Am. J. Clin. Nutr. 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Kelemen, L.E.; Kushi, L.H.; Jacobs, D.R.; Cerhan, J.R. Associations of Dietary Protein with Disease and Mortality in a Prospective Study of Postmenopausal Women. Am. J. Epidemiol. 2005, 161, 239–249. [Google Scholar] [CrossRef]
- Fung, T.T. Low-Carbohydrate Diets and All-Cause and Cause-Specific Mortality. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red Meat Consumption and Mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Sakak, F.; Maroofi, M.; Rahmani, J.; Bellissimo, N.; Hekmatdoost, A. Serum uric acid and risk of cardiovascular mortality: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc. Disord. 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Igl, W.; Kamal-Eldin, A.; Johansson, Å.; Liebisch, G.; Gnewuch, C.; Schmitz, G.; Gyllensten, U. Animal source food intake and association with blood cholesterol, glycerophospholipids and sphingolipids in a northern Swedish population. Int. J. Circumpolar Health 2013, 72. [Google Scholar] [CrossRef] [Green Version]
- Päivärinta, E.; Itkonen, S.T.; Pellinen, T.; Lehtovirta, M.; Erkkola, M.; Pajari, A.-M. Replacing Animal-Based Proteins with Plant-Based Proteins Changes the Composition of a Whole Nordic Diet—A Randomised Clinical Trial in Healthy Finnish Adults. Nutrients 2020, 12, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Song, A.; Zheng, C.; Wang, M.; Song, G. Effects of plant protein and animal protein on lipid profile, body weight and body mass index on patients with hypercholesterolemia: A systematic review and meta-analysis. Acta Diabetol. 2020, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; Potter, S.M.; Weigel, R.; Hannum, S.; Erdman, J.W.; Hasler, C.M. Effects of feeding 4 levels of soy protein for 3 and 6 wk on blood lipids and apolipoproteins in moderately hypercholesterolemic men. Am. J. Clin. Nutr. 2000, 71, 1077–1084. [Google Scholar] [CrossRef]
- Hruby, A.; Jacques, P.F. Dietary protein and changes in markers of cardiometabolic health across 20 years of follow-up in middle-aged Americans. Public Health Nutr. 2018, 21, 2998–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berryman, C.E.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L.; Pasiakos, S.M. Diets higher in animal and plant protein are associated with lower adiposity and do not impair kidney function in US adults. Am. J. Clin. Nutr. 2016, 104, 743–749. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Azzout-Marniche, D.; Arfsten, J.; Egli, L.; Gaudichon, C.; Karagounis, L.G.; Tomé, D. A Systematic Review of the Effects of Plant Compared with Animal Protein Sources on Features of Metabolic Syndrome. J. Nutr. 2017, 147, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Sucher, S.; Markova, M.; Hornemann, S.; Pivovarova, O.; Rudovich, N.; Thomann, R.; Schneeweiss, R.; Rohn, S.; Pfeiffer, A.F.H. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: A randomized clinical trial. Diabetes Obes. Metab. 2017, 19, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P.; Virtanen, H.E.; Voutilainen, S. Egg consumption and risk of incident type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2015, 101, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
- Tracy, C.R.; Best, S.; Bagrodia, A.; Poindexter, J.R.; Adams-Huet, B.; Sakhaee, K.; Maalouf, N.; Pak, C.Y.; Pearle, M.S. Animal Protein and the Risk of Kidney Stones: A Comparative Metabolic Study of Animal Protein Sources. J. Urol. 2014, 192, 137–141. [Google Scholar] [CrossRef]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef]
- Liang, W.; Wang, L.; Guo, D.; Nie, Z.; Chen, Y.; Jin, Y.; He, L.; Yao, Y. Blood lipid profile and glucose of university students (China). Nutr. Hosp. 2015, 31, 2182–2186. [Google Scholar] [PubMed]
- Chinese Center For Disease Control And Prevention (China CDC). 2015 China Health and Nutrition Survey Project Launched. Available online: http://www.chinacdc.cn/zxdt/201511/t20151123_122181.html (accessed on 11 November 2020).
- Zhang, B.; Zhai, F.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Su, C.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, B. Secular Trends in Energy and Macronutrient Intakes and Distribution among Adult Females (1991–2015): Results from the China Health and Nutrition Survey. Nutrients 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, Y.-T.; Zheng, W.; Shu, X.-O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Zhao, J.; Wu, Y.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B. Temporal Trends in Dietary Macronutrient Intakes among Adults in Rural China from 1991 to 2011: Findings from the CHNS. Nutrients 2017, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition Table, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Yang, Y.X.; He, M.; Pan, X.C. China Food Composition Table 2004; Peking University Medical Press: Beijing, China, 2004. [Google Scholar]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition Table 2002; Peking University Medical Press: Beijing, China, 2002. [Google Scholar]
- Virtanen, J.K.; Koskinen, T.T.; Voutilainen, S.; Mursu, J.; Tuomainen, T.-P.; Kokko, P.; Virtanen, J.K. Intake of different dietary proteins and risk of type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2017, 117, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Yan, S.; Li, J.; Li, S.; Zhang, B.; Du, S.; Gordon-Larsen, P.; Adair, L.; Popkin, B.M. The expanding burden of cardiometabolic risk in China: The China Health and Nutrition Survey. Obes. Rev. 2012, 13, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Cormier, H.; Thifault, E.; Garneau, V.; Tremblay, A.; Drapeau, V.; Pérusse, L.; Vohl, M.-C. Association between yogurt consumption, dietary patterns, and cardio-metabolic risk factors. Eur. J. Nutr. 2015, 55, 577–587. [Google Scholar] [CrossRef]
- Working Group on Obesity in China. Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults. Acta Nutr. Sin. 2004, 26, 1–4. [Google Scholar] [CrossRef]
- Qian, X.; Su, C.; Zhang, B.; Qin, G.; Wang, H.; Wu, Z. Changes in distributions of waist circumference, waist-to-hip ratio and waist-to-height ratio over an 18-year period among Chinese adults: A longitudinal study using quantile regression. BMC Public Health 2019, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Sun, X.; Huo, R.; Yu, X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: The China Health and Nutrition Survey 2009. Int. J. Obes. 2013, 38, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, A.; Leblanc, C.; Lortie, G.; Savard, R.; Thériault, G. A method to assess energy expenditure in children and adults. Am. J. Clin. Nutr. 1983, 37, 461–467. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Wu, J.; Wang, R.; Mao, D.; Chen, J.; Li, W.; Yang, Y.; Piao, J.; Yang, L.; et al. Prevalence and Risk Factors for Anemia in Non-pregnant Childbearing Women from the Chinese Fifth National Health and Nutrition Survey. Int. J. Environ. Res. Public Health 2019, 16, 1290. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.M. Clinical Laboratory Normal Reference Value Manual; Military Medical Science Press: Beijing, China, 2002. [Google Scholar]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant Protein and Animal Proteins: Do They Differentially Affect Cardiovascular Disease Risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef]
- Matthan, N.R.; Jalbert, S.M.; Ausman, L.M.; Kuvin, J.T.; Karas, R.H.; Lichtenstein, A.H. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Srichaikul, K.; Wong, J.M.W.; Kendall, C.; Bashyam, B.; Vidgen, E.; Lamarche, B.; Rao, A.V.; Jones, P.J.H.; Josse, R.G.; et al. Supplemental Barley Protein and Casein Similarly Affect Serum Lipids in Hypercholesterolemic Women and Men. J. Nutr. 2010, 140, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Tierney, K.; Adams-Huet, B.; Boonyavarakul, A.; Jacob, K.; Quittner, C.; Dinges, W.; Peterson, D.; Garg, A. The role of diet, exercise and smoking in dyslipidaemia in HIV-infected patients with lipodystrophy. HIV Med. 2005, 6, 291–298. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Ghasemi, A.; Azizi, F. Contribution of dietary amino acids composition to incidence of cardiovascular outcomes: A prospective population-based study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 633–641. [Google Scholar] [CrossRef]
- Giroux, I.; Kurowska, E.M.; Carroll, K.K. Role of dietary lysine, methionine, and arginine in the regulation of hypercholesterolemia in rabbits. J. Nutr. Biochem. 1999, 10, 166–171. [Google Scholar] [CrossRef]
- Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Doroszko, A. Intraplatelet L-Arginine-Nitric Oxide Metabolic Pathway: From Discovery to Clinical Implications in Prevention and Treatment of Cardiovascular Disorders. Oxidative Med. Cell. Longev. 2020, 2020, 1015908. [Google Scholar] [CrossRef] [PubMed]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmed, M.I.; Zou, X.; Hussain, M.; Zhang, M.; Zhao, F.; Xu, X.; Zhou, G.; Li, C. Beef, Casein, and Soy Proteins Differentially Affect Lipid Metabolism, Triglycerides Accumulation and Gut Microbiota of High-Fat Diet-Fed C57BL/6J Mice. Front. Microbiol. 2018, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowles, R.L.; Lee, J.-Y.; Gallaher, D.D.; Stuefer-Powell, C.L.; Carr, T.P. Dietary Stearic Acid Alters Gallbladder Bile Acid Composition in Hamsters Fed Cereal-Based Diets. J. Nutr. 2002, 132, 3119–3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Piscitelli, D.; Horowitz, M.; Jones, K.L.; Clifton, P.M.; Standfield, S.; Hausken, T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am. J. Clin. Nutr. 2015, 102, 1574–1584. [Google Scholar] [CrossRef]
- Sun, L.; Ranawana, D.V.; Leow, M.K.-S.; Henry, C.J. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur. J. Nutr. 2014, 53, 1719–1726. [Google Scholar] [CrossRef]
- Shi, Z.; Ganji, V. Dietary patterns and cardiovascular disease risk among Chinese adults: A prospective cohort study. Eur. J. Clin. Nutr. 2020, 74, 1725–1735. [Google Scholar] [CrossRef]
- Møller, G.; Ritz, C.; Dragsted, L.O.; Larsen, T.; Raben, A.; Sluik, D.; Dragsted, L.O.; Larsen, T.M.; Poppitt, S.D.; Silvestre, M.P.; et al. A Protein Diet Score, Including Plant and Animal Protein, Investigating the Association with HbA1c and eGFR—The PREVIEW Project. Nutrients 2017, 9, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juraschek, S.P.; McAdams-DeMarco, M.A.; Gelber, A.C.; Sacks, F.M.; Appel, L.J.; White, K.J.; Iii, E.R.M. Effects of Lowering Glycemic Index of Dietary Carbohydrate on Plasma Uric Acid Levels: The OmniCarb Randomized Clinical Trial. Arthritis Rheumatol. 2015, 68, 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, C.; Choi, H.; E Chaisson, C.; Hunter, D.J.; Niu, J.; Neogi, T. Purine-rich foods intake and recurrent gout attacks. Ann. Rheum. Dis. 2012, 71, 1448–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.K.; Liu, S.; Curhan, G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005, 52, 283–289. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Participants | Animal Protein | Plant Protein | ||||
|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | ||
| Median of each quintile,% of total energy | - | 0.8 | 4.2 | 8.9 | 6.3 | 8.9 | 12.3 |
| Number or participants, n | 7886 | 1571 | 1569 | 1631 | 1553 | 1591 | 1656 |
| Age, y | 50 ± 15 | 52 ± 15 | 50 ± 15 | 48 ± 15 | 49 ± 15 | 50 ± 15 | 51 ± 14 |
| Sex, n (%) | |||||||
| Women | 4196 (53.2) | 869 (55.3) | 860 (54.8) | 822 (50.4) | 816 (52.5) | 837 (52.6) | 902 (54.5) |
| Men | 3690 (46.8) | 702 (44.7) | 709 (45.2) | 809 (49.6) | 737 (47.5) | 754 (47.4) | 754 (45.5) |
| BMI, kg/m2 | 23.3 ± 3.4 | 23.3 ± 3.3 | 23.2 ± 3.4 | 23.2 ± 3.5 | 23.1 ± 3.3 | 23.3 ± 3.5 | 23.6 ± 3.4 |
| Waist to hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Urban index, n (%) | |||||||
| Low | 2623 (33.3) | 1022 (65.1) | 435 (27.7) | 212 (13.0) | 249 (16.0) | 465 (29.2) | 889 (53.7) |
| Medium | 2600 (33.0) | 422 (26.9) | 641 (40.9) | 427 (26.2) | 548 (35.3) | 562 (35.3) | 426 (25.7) |
| High | 2663 (33.8) | 127 (8.1) | 493 (31.4) | 992 (60.8) | 756 (48.7) | 564 (35.4) | 341 (20.6) |
| Region, n (%) | |||||||
| Northern | 3311 (42.0) | 1012 (64.4) | 619 (39.5) | 396 (24.3) | 435 (28.0) | 646 (40.6) | 991 (59.8) |
| Southern | 4575 (58.0) | 559 (35.6) | 950 (60.5) | 1235 (75.7) | 1118 (72.0) | 945 (59.4) | 665 (40.2) |
| Higher education, n (%) | 1901 (24.1) | 187 (11.9) | 348 (22.2) | 625 (38.3) | 484 (31.2) | 398 (25.0) | 285 (17.2) |
| Alcohol intake, n (%) | 2601 (33.0) | 451 (28.7) | 499 (31.8) | 647 (39.7) | 540 (34.8) | 541 (34.0) | 519 (31.3) |
| Current smokers, n (%) | 2214 (28.1) | 466 (29.7) | 424 (27.0) | 490 (30.0) | 441 (28.4) | 441 (27.7) | 453 (27.4) |
| Physical activity level, MET-h/week | 69.0 ± 100.1 | 97.4 ± 118.5 | 66.5 ± 97.3 | 46.3 ± 81.3 | 53.7 ± 86.7 | 67.8 ± 99.9 | 81.3 ± 110.4 |
| SBP, mmHg | 124.3 ± 18.7 | 124.7 ± 18.4 | 125.0 ± 19.3 | 123.0 ± 18.2 | 123.6 ± 19.0 | 124.3 ± 18.6 | 125.7 ± 17.6 |
| DBP, mmHg | 80.1 ± 11.1 | 81.1 ± 10.8 | 79.6 ± 11.2 | 79.7 ± 11.2 | 79.3 ± 11.1 | 80.1 ± 11.4 | 81.7 ± 10.8 |
| Dietary intakes | |||||||
| Total energy, kcal/day | 1729.7 ± 14.5 | 1729.2 ± 9.6 | 1729.7 ± 13.6 | 1731.0 ± 19.7 | 1730.5 ± 22.2 | 1729.7 ± 11.4 | 1728.6 ± 10.4 |
| Protein, % of total energy | 14.4 ± 3.0 | 12.4 ± 2.1 | 13.8 ± 2.1 | 17.8 ± 2.8 | 14.3 ± 3.3 | 14.1 ± 2.7 | 15.4 ± 3.1 |
| Animal protein,% of total energy | 4.7 ± 3.3 | 0.8 ± 0.6 | 4.2 ± 0.5 | 9.7 ± 2.5 | 7.2 ± 3.5 | 4.8 ± 2.7 | 2.5 ± 2.4 |
| Plant protein, % of total energy | 9.2 ± 2.5 | 11.1 ± 2.4 | 9.0 ± 2.2 | 7.6 ± 2.1 | 6.0 ± 1.0 | 8.9 ± 0.4 | 12.8 ± 1.9 |
| Animal to plant protein ratio | 0.6 ± 0.6 | 0.1 ± 0.1 | 0.5 ± 0.1 | 1.4 ± 0.6 | 1.3 ± 0.7 | 0.5 ± 0.3 | 0.2 ± 0.2 |
| Fat, % of total energy | 18.0 ± 9.2 | 9.0 ± 6.4 | 19.2 ± 7.0 | 24.7 ± 8.7 | 25.9 ± 8.7 | 17.3 ± 7.6 | 12.4 ± 7.9 |
| SFA, % of total energy | 4.9 ± 3.0 | 1.9 ± 1.7 | 5.2 ± 2.2 | 7.3 ± 3.0 | 7.7 ± 3.0 | 4.7 ± 2.4 | 2.9 ± 2.2 |
| MUFA, % of total energy | 7.1 ± 4.5 | 2.6 ± 2.6 | 7.8 ± 3.6 | 10.1 ± 4.5 | 11.2 ± 4.5 | 6.8 ± 3.6 | 3.8 ± 3.4 |
| PUFA, % of total energy | 2.3 ± 1.6 | 1.6 ± 1.6 | 2.3 ± 1.4 | 2.9 ± 1.5 | 2.5 ± 1.2 | 2.1 ± 1.4 | 2.7 ± 2.2 |
| Cholesterol, % of total energy | 0.1 ± 0.1 | 0 ± 0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Carbohydrates, % of total energy | 67.6 ± 10.5 | 78.6 ± 7.0 | 67.1 ± 7.1 | 57.6 ± 9.0 | 59.7 ± 9.8 | 68.5 ± 9.2 | 72.2 ± 10.2 |
| Fiber, g/day | 10.9 ± 5.3 | 12.2 ± 5.2 | 10.4 ± 4.9 | 10.3 ± 5.4 | 9.6 ± 5.4 | 10.6 ± 5.2 | 12.8 ± 5.1 |
| CMRF concentrations | |||||||
| TG, mmol/L | 1.6 ± 1.2 | 1.5 ± 1.1 | 1.6 ± 1.3 | 1.7 ± 1.3 | 1.6 ± 1.3 | 1.6 ± 1.2 | 1.6 ± 1.2 |
| TC, mmol/L | 4.9 ± 1.0 | 4.7 ± 1.0 | 4.9 ± 1.0 | 5.0 ± 1.0 | 4.9 ± 1.0 | 4.8 ± 1.0 | 4.8 ± 1.0 |
| HDL-C, mmol/L | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.4 |
| LDL-C, mmol/L | 3.0 ± 1.0 | 2.9 ± 1.1 | 3.0 ± 0.9 | 3.0 ± 1.0 | 3.0 ± 1.0 | 3.0 ± 1.0 | 2.9 ± 1.1 |
| Non-HDL-C, mmol/L | 3.5 ± 1.0 | 3.3 ± 1.0 | 3.5 ± 1.0 | 3.6 ± 1.0 | 3.5 ± 1.0 | 3.5 ± 1.0 | 3.4 ± 1.0 |
| TC:HDL-C | 3.6 ± 1.2 | 3.5 ± 1.1 | 3.6 ± 1.1 | 3.7 ± 1.2 | 3.6 ± 1.1 | 3.6 ± 1.2 | 3.6 ± 1.2 |
| LDL-C:HDL-C | 2.2 ± 0.8 | 2.2 ± 0.9 | 2.2 ± 0.8 | 2.3 ± 0.9 | 2.2 ± 0.8 | 2.2 ± 0.9 | 2.2 ± 0.9 |
| Lipoprotein (a) (mg/L) | 133.0 ± 149.2 | 141.3 ± 149.7 | 132.2 ± 152.1 | 129.0 ± 150.7 | 133.9 ± 155.7 | 132.6 ± 154.1 | 137.0 ± 148.3 |
| Glucose (mmol/L) | 5.3 ± 1.2 | 5.2 ± 1.3 | 5.3 ± 1.2 | 5.3 ± 1.2 | 5.3 ± 1.2 | 5.3 ± 1.2 | 5.3 ± 1.3 |
| Insulin (μIU/mL) | 13.8 ± 19.3 | 13.8 ± 21.5 | 13.3 ± 12.6 | 14.4 ± 19.1 | 13.7 ± 13.6 | 14.1 ± 21.9 | 14.1 ± 21.3 |
| HOMA-IR | 3.5 ± 6.0 | 3.5 ± 7.2 | 3.4 ± 4.4 | 3.6 ± 5.6 | 3.5 ± 5.2 | 3.6 ± 7.5 | 3.5 ± 5.6 |
| HbA1c (%) | 5.6 ± 0.8 | 5.7 ± 0.8 | 5.5 ± 0.8 | 5.5 ± 0.7 | 5.5 ± 0.8 | 5.6 ± 0.8 | 5.7 ± 0.8 |
| hsCRP (mg/L) | 2.5 ± 6.8 | 2.4 ± 5.4 | 2.7 ± 8.3 | 2.1 ± 3.8 | 2.3 ± 5.5 | 2.5 ± 6.9 | 2.3 ± 5.0 |
| Uric acid (μmol/L) | 306.4 ± 98.1 | 286.9 ± 90.7 | 303.4 ± 97.9 | 325.5 ± 103.4 | 315.3 ± 99.4 | 307.3 ± 99.6 | 297.3 ± 96.9 |
| Nutrients | Food Sources | Percentage of Contribution to Total Intake (%) |
|---|---|---|
| Total protein | Grains | 45.7 |
| Red meat | 15.7 | |
| Legumes | 9.2 | |
| Fruits and vegetables | 8.2 | |
| Egg | 5.6 | |
| Fish and seafood | 5.5 | |
| Animal protein | Red meat | 48.8 |
| Egg | 24.7 | |
| Fish and seafood | 15.5 | |
| White meat | 7.7 | |
| Offal | 1.8 | |
| Dairy | 1.5 | |
| Plant protein | Grains | 68.9 |
| Legumes | 13.8 | |
| Fruits and vegetables | 13.1 | |
| Tubers | 2.0 | |
| Nuts | 1.4 | |
| Fungi and algae | 0.7 |
| CMRF | Total Protein | Animal Protein | Plant Protein | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Lipid and lipoprotein profiles | ||||||
| TG (mmol/L) | ||||||
| Model 1 | 0.08 (0.03, 0.12) | <0.01 | 0.07 (0.03, 0.11) | <0.01 | −0.01 (−0.07, 0.04) | 0.63 |
| Model 2 | 0 (−0.06, 0.06) | 0.96 | 0.05 (−0.01, 0.12) | 0.11 | −0.02 (−0.10, 0.05) | 0.58 |
| TC (mmol/L) | ||||||
| Model 1 | 0.11 (0.07, 0.14) | <0.01 | 0.12 (0.09, 0.16) | <0.01 | −0.09 (−0.13, −0.04) | <0.01 |
| Model 2 | 0.05 (0, 0.10) | 0.03 | 0.09 (0.04, 0.15) | <0.01 | −0.04 (−0.10, 0.02) | 0.22 |
| HDL-C (mmol/L) | ||||||
| Model 1 | 0 (−0.01, 0.01) | 0.79 | 0 (−0.01, 0.01) | 0.85 | 0 (−0.01, 0.02) | 0.92 |
| Model 2 | 0.02 (0, 0.04) | 0.04 | 0 (−0.02, 0.02) | 0.72 | 0.02 (0, 0.05) | 0.03 |
| LDL-C (mmol/L) | ||||||
| Model 1 | 0.06 (0.02, 0.09) | <0.01 | 0.07 (0.04, 0.10) | <0.01 | −0.07 (−0.11, −0.03) | <0.01 |
| Model 2 | 0.03 (−0.02, 0.08) | 0.26 | 0.05 (0, 0.11) | 0.04 | −0.06 (−0.12, 0) | 0.07 |
| Non-HDL-C (mmol/L) | ||||||
| Model 1 | 0.12 (0.08, 0.16) | <0.01 | 0.13 (0.10, 0.17) | <0.01 | −0.08 (−0.13, −0.04) | <0.01 |
| Model 2 | 0.03 (−0.02, 0.08) | 0.31 | 0.08 (0.02, 0.13) | <0.01 | −0.06 (−0.13, 0) | <0.05 |
| TC:HDL-C | ||||||
| Model 1 | 0.11 (0.07, 0.15) | <0.01 | 0.10 (0.06, 0.14) | <0.01 | −0.05 (−0.10, 0.01) | 0.08 |
| Model 2 | 0.01 (−0.05, 0.06) | 0.82 | 0.05 (−0.01, 0.12) | 0.08 | −0.05 (−0.12, 0.02) | 0.17 |
| LDL-C:HDL-C | ||||||
| Model 1 | 0.05 (0.02, 0.08) | <0.01 | 0.06 (0.03, 0.09) | <0.01 | −0.05 (−0.09, −0.01) | <0.01 |
| Model 2 | 0 (−0.04, 0.04) | 0.90 | 0.04 (−0.01, 0.08) | 0.12 | −0.06 (−0.12, −0.01) | 0.01 |
| Lipoprotein (a) (mg/L) | ||||||
| Model 1 | −2.85 (−8.50, 2.79) | 0.32 | −4.67 (−9.74, 0.41) | 0.07 | 5.94 (−0.70, 12.58) | 0.08 |
| Model 2 | 2.59 (−5.28, 10.45) | 0.52 | 5.82 (−2.67, 14.31) | 0.18 | −0.86 (−10.60, 8.89) | 0.86 |
| Glucose homeostasis biomarkers | ||||||
| Glucose (mmol/L) | ||||||
| Model 1 | 0.02 (−0.03, 0.06) | 0.44 | 0.04 (0, 0.08) | 0.04 | −0.08 (−0.13, −0.02) | <0.01 |
| Model 2 | −0.02 (−0.08, 0.04) | 0.55 | −0.05 (−0.12, 0.02) | 0.13 | −0.02 (−0.10, 0.06) | 0.58 |
| Insulin (μIU/mL) | ||||||
| Model 1 | 0.66 (−0.06, 1.37) | 0.07 | 0.33 (−0.32, 0.97) | 0.32 | 0.09 (−0.76, 0.93) | 0.84 |
| Model 2 | 0.25 (−0.74, 1.24) | 0.62 | −0.17 (−1.24, 0.91) | 0.76 | 0.38 (−0.85, 1.62) | 0.54 |
| HOMA-IR | ||||||
| Model 1 | 0.12 (−0.10, 0.35) | 0.28 | 0.06 (−0.14, 0.26) | 0.57 | −0.06 (−0.32, 0.20) | 0.65 |
| Model 2 | −0.03 (−0.34, 0.28) | 0.83 | −0.19 (−0.53, 0.14) | 0.26 | −0.05 (−0.43, 0.33) | 0.80 |
| HbA1c (%) | ||||||
| Model 1 | 0.02 (−0.01, 0.05) | 0.14 | −0.07 (−0.10, −0.05) | <0.01 | 0.13 (0.09, 0.16) | <0.01 |
| Model 2 | 0.03 (−0.01, 0.07) | 0.18 | −0.03 (−0.08, 0.01) | 0.13 | 0.07 (0.02, 0.12) | <0.01 |
| hsCRP (mg/L) | ||||||
| Model 1 | −0.13 (−0.38, 0.13) | 0.33 | −0.09 (−0.31, 0.14) | 0.45 | 0.02 (−0.28, 0.32) | 0.89 |
| Model 2 | −0.20 (−0.55, 0.15) | 0.25 | −0.26 (−0.64, 0.11) | 0.17 | −0.07 (−0.50, 0.37) | 0.77 |
| Uric acid (μmol/L) | ||||||
| Model 1 | 16.45 (12.81, 20.09) | <0.01 | 20.80 (17.54, 24.07) | <0.01 | −12.26 (−16.56, −7.96) | <0.01 |
| Model 2 | 7.44 (3.11, 11.78) | <0.01 | 15.32 (10.63, 20.01) | <0.01 | −5.25 (−10.60, 0.11) | 0.05 |
| CMRF | Total protein | Animal protein | Plant protein | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Lipid and lipoprotein profiles | ||||||
| TG (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0.04 (−0.03, 0.11) | 0.23 | 0.08 (0.01, 0.16) | 0.02 | −0.02 (−0.10, 0.07) | 0.70 |
| BMI ≥ 24 kg/m2 | −0.06 (−0.18, 0.06) | 0.29 | 0.01 (−0.13, 0.14) | 0.94 | −0.03 (−0.17, 0.11) | 0.67 |
| TC (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0.08 (0.02, 0.14) | 0.01 | 0.12 (0.05, 0.18) | <0.01 | −0.03 (−0.11, 0.05) | 0.46 |
| BMI ≥ 24 kg/m2 | 0.03 (−0.06, 0.11) | 0.54 | 0.05 (−0.04, 0.14) | 0.27 | −0.03 (−0.13, 0.07) | 0.51 |
| HDL-C (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0.02 (0, 0.05) | 0.04 | 0 (−0.02, 0.03) | 0.77 | 0.04 (0.01, 0.07) | 0.01 |
| BMI ≥ 24 kg/m2 | 0.01 (−0.02, 0.03) | 0.62 | 0 (−0.03, 0.03) | 0.85 | 0 (−0.03, 0.04) | 0.84 |
| LDL-C (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0.04 (−0.02, 0.10) | 0.22 | 0.08 (0.01, 0.14) | 0.02 | −0.07 (−0.15, 0.01) | 0.07 |
| BMI ≥ 24 kg/m2 | 0.02 (−0.06, 0.10) | 0.62 | 0.02 (−0.07, 0.11) | 0.68 | −0.02 (−0.11, 0.08) | 0.73 |
| Non-HDL-C (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0.04 (−0.02, 0.11) | 0.18 | 0.10 (0.03, 0.17) | <0.01 | −0.07 (−0.15, 0.02) | 0.11 |
| BMI ≥ 24 kg/m2 | 0.01 (−0.07, 0.10) | 0.78 | 0.04 (−0.05, 0.14) | 0.36 | −0.05 (−0.15, 0.05) | 0.36 |
| TC:HDL-C | ||||||
| BMI < 24 kg/m2 | 0 (−0.06, 0.07) | 0.93 | 0.07 (0, 0.14) | 0.04 | −0.10 (−0.18, −0.01) | 0.02 |
| BMI ≥ 24 kg/m2 | 0.02 (−0.08, 0.13) | 0.67 | 0.03 (−0.09, 0.14) | 0.65 | 0.03 (−0.09, 0.15) | 0.65 |
| LDL-C:HDL-C | ||||||
| BMI < 24 kg/m2 | −0.01 (−0.06, 0.04) | 0.76 | 0.05 (−0.01, 0.11) | 0.08 | −0.11 (−0.17, −0.04) | <0.01 |
| BMI ≥ 24 kg/m2 | 0.02 (−0.05, 0.09) | 0.64 | 0.01 (−0.06, 0.09) | 0.73 | 0.01 (−0.08, 0.09) | 0.84 |
| Lipoprotein (a) (mg/L) | ||||||
| BMI < 24 kg/m2 | 2.38 (−8.04, 12.81) | 0.65 | 7.01 (−4.17, 18.19) | 0.22 | 2.19 (−11.09, 15.46) | 0.75 |
| BMI ≥ 24 kg/m2 | 2.22 (−9.72, 14.16) | 0.72 | 3.85 (−9.18, 16.88) | 0.56 | −5.38 (−19.62, 8.85) | 0.46 |
| Glucose homeostasis biomarkers | ||||||
| Glucose (mmol/L) | ||||||
| BMI < 24 kg/m2 | 0 (−0.07, 0.07) | 1.00 | −0.02 (−0.09, 0.05) | 0.59 | −0.03 (−0.12, 0.06) | 0.46 |
| BMI ≥ 24 kg/m2 | −0.05 (−0.16, 0.07) | 0.44 | −0.10 (−0.23, 0.03) | 0.12 | 0 (−0.13, 0.14) | 0.95 |
| Insulin (μIU/mL) | ||||||
| BMI < 24 kg/m2 | 0.34 (−1.05, 1.73) | 0.63 | −0.22 (−1.71, 1.28) | 0.78 | 0.40 (−1.37, 2.17) | 0.66 |
| BMI ≥ 24 kg/m2 | 0.24 (−1.10, 1.59) | 0.72 | −0.07 (−1.54, 1.41) | 0.93 | 0.59 (−1.01, 2.18) | 0.47 |
| HOMA-IR | ||||||
| BMI < 24 kg/m2 | 0.03 (−0.38, 0.43) | 0.90 | −0.14 (−0.57, 0.29) | 0.52 | −0.10 (−0.61, 0.41) | 0.69 |
| BMI ≥ 24 kg/m2 | −0.12 (−0.61, 0.37) | 0.64 | −0.30 (−0.84, 0.23) | 0.27 | 0.07 (−0.51, 0.66) | 0.81 |
| HbA1c (%) | ||||||
| BMI < 24 kg/m2 | 0.03 (−0.02, 0.08) | 0.19 | −0.02 (−0.07, 0.03) | 0.49 | 0.06 (0, 0.13) | <0.05 |
| BMI ≥ 24 kg/m2 | 0.02 (−0.05, 0.09) | 0.58 | −0.06 (−0.13, 0.02) | 0.14 | 0.08 (0, 0.16) | <0.05 |
| hsCRP (mg/L) | ||||||
| BMI < 24 kg/m2 | −0.28 (−0.77, 0.21) | 0.27 | −0.38 (−0.90, 0.15) | 0.16 | 0.07 (−0.56, 0.70) | 0.82 |
| BMI ≥ 24 kg/m2 | −0.07 (−0.53, 0.38) | 0.75 | −0.05 (−0.55, 0.45) | 0.85 | −0.26 (−0.80, 0.29) | 0.36 |
| Uric acid (μmol/L) | ||||||
| BMI < 24 kg/m2 | 8.35 (3.16, 13.54) | <0.01 | 15.82 (10.27, 21.38) | <0.01 | −5.86 (−12.45, 0.74) | 0.08 |
| BMI ≥ 24 kg/m2 | 6.82 (−0.87, 14.51) | 0.08 | 14.91 (6.46, 23.36) | <0.01 | −3.82 (−12.93, 5.30) | 0.41 |
| CMRF | Total Protein | Animal Protein | Plant Protein | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Lipid and lipoprotein profiles | ||||||
| TG (mmol/L) | ||||||
| Women | 0.02 (−0.06, 0.10) | 0.60 | 0.06 (−0.03, 0.14) | 0.17 | −0.01 (−0.10, 0.08) | 0.87 |
| Men | −0.04 (−0.14, 0.06) | 0.45 | 0.03 (−0.07, 0.14) | 0.53 | −0.04 (−0.16, 0.09) | 0.55 |
| TC (mmol/L) | ||||||
| Women | 0.06 (0, 0.13) | 0.07 | 0.08 (0, 0.15) | 0.04 | 0 (−0.08, 0.08) | 0.95 |
| Men | 0.03 (−0.04, 0.10) | 0.37 | 0.09 (0.02, 0.17) | 0.02 | −0.09 (−0.18, 0) | <0.05 |
| HDL-C (mmol/L) | ||||||
| Women | 0.01 (−0.01, 0.03) | 0.43 | −0.01 (−0.04, 0.02) | 0.41 | 0.01 (−0.02, 0.04) | 0.56 |
| Men | 0.03 (0, 0.05) | 0.03 | 0.02 (−0.01, 0.05) | 0.18 | 0.04 (0.01, 0.08) | 0.01 |
| LDL-C (mmol/L) | ||||||
| Women | 0.06 (−0.01, 0.12) | 0.10 | 0.05 (−0.02, 0.13) | 0.16 | 0.03 (−0.05, 0.11) | 0.52 |
| Men | −0.01 (−0.08, 0.06) | 0.78 | 0.04 (−0.03, 0.12) | 0.25 | −0.17 (−0.26, −0.07) | <0.01 |
| Non-HDL-C (mmol/L) | ||||||
| Women | 0.04 (−0.03, 0.11) | 0.26 | 0.07 (−0.01, 0.15) | 0.08 | 0 (−0.09, 0.08) | 0.97 |
| Men | 0 (−0.07, 0.08) | 0.93 | 0.07 (−0.01, 0.14) | 0.09 | −0.14 (−0.23, −0.04) | <0.01 |
| TC:HDL-C | ||||||
| Women | 0.03 (−0.04, 0.10) | 0.44 | 0.08 (0, 0.16) | 0.05 | 0 (−0.09, 0.08) | 0.96 |
| Men | −0.03 (−0.12, 0.06) | 0.50 | 0.02 (−0.08, 0.11) | 0.74 | −0.11 (−0.22, 0) | <0.05 |
| LDL-C:HDL-C | ||||||
| Women | 0.03 (−0.02, 0.09) | 0.23 | 0.05 (−0.01, 0.12) | 0.08 | 0.02 (−0.04, 0.09) | 0.47 |
| Men | −0.05 (−0.11, 0.01) | 0.13 | 0.01 (−0.06, 0.07) | 0.84 | −0.18 (−0.26, −0.10) | <0.01 |
| Lipoprotein (a) (mg/L) | ||||||
| Women | −1.05 (−12.32, 10.21) | 0.85 | 3.49 (−9.00, 15.98) | 0.58 | −2.26 (−15.92, 11.39) | 0.75 |
| Men | 6.22 (−4.73, 17.18) | 0.27 | 7.52 (−4.01, 19.06) | 0.20 | 1.06 (−12.86, 14.98) | 0.88 |
| Glucose homeostasis biomarkers | ||||||
| Glucose (mmol/L) | ||||||
| Women | −0.04 (−0.12, 0.04) | 0.30 | −0.05 (−0.13, 0.04) | 0.27 | −0.04 (−0.13, 0.06) | 0.45 |
| Men | 0 (−0.10, 0.10) | 0.94 | −0.05 (−0.15, 0.06) | 0.37 | −0.01 (−0.13, 0.12) | 0.91 |
| Insulin (μIU/mL) | ||||||
| Women | 0.84 (−0.49, 2.16) | 0.22 | 0.63 (−0.85, 2.11) | 0.40 | 0.18 (−1.43, 1.79) | 0.83 |
| Men | −0.49 (−2.00, 1.01) | 0.52 | −0.96 (−2.54, 0.63) | 0.24 | 0.48 (−1.43, 2.39) | 0.62 |
| HOMA-IR | ||||||
| Women | 0.10 (−0.34, 0.55) | 0.66 | −0.01 (−0.51, 0.48) | 0.96 | −0.10 (−0.64, 0.44) | 0.73 |
| Men | −0.21 (−0.64, 0.22) | 0.34 | −0.37 (−0.83, 0.08) | 0.10 | −0.03 (−0.58, 0.51) | 0.91 |
| HbA1c (%) | ||||||
| Women | 0.02 (−0.03, 0.08) | 0.44 | −0.04 (−0.10, 0.03) | 0.26 | 0.06 (0, 0.13) | 0.06 |
| Men | 0.03 (−0.02, 0.09) | 0.25 | −0.03 (−0.08, 0.03) | 0.41 | 0.08 (0.01, 0.15) | 0.03 |
| hsCRP (mg/L) | ||||||
| Women | −0.23 (−0.64, 0.18) | 0.26 | −0.11 (−0.57, 0.35) | 0.64 | −0.18 (−0.68, 0.32) | 0.48 |
| Men | −0.16 (−0.74, 0.42) | 0.59 | −0.38 (−1.00, 0.23) | 0.22 | 0.07 (−0.67, 0.81) | 0.85 |
| Uric acid (μmol/L) | ||||||
| Women | 7.25 (2.09, 12.42) | <0.01 | 14.81 (9.05, 20.56) | <0.01 | −4.01 (−10.23, 2.22) | 0.21 |
| Men | 6.76 (−0.30, 13.83) | 0.06 | 14.65 (7.22, 22.07) | <0.01 | −6.79 (−15.75, 2.18) | 0.14 |
| CMRF | Total Protein | Animal Protein | Plant Protein | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Lipid and lipoprotein profiles | ||||||
| TG (mmol/L) | ||||||
| Age < 60 years | 0.01 (−0.07, 0.08) | 0.85 | 0.08 (0, 0.16) | 0.05 | 0.01 (−0.08, 0.10) | 0.86 |
| Age ≥ 60 years | −0.01 (−0.12, 0.10) | 0.86 | −0.01 (−0.13, 0.11) | 0.90 | −0.07 (−0.20, 0.06) | 0.28 |
| TC (mmol/L) | ||||||
| Age < 60 years | 0.05 (−0.01, 0.10) | 0.12 | 0.09 (0.03, 0.15) | <0.01 | −0.02 (−0.09, 0.06) | 0.65 |
| Age ≥ 60 years | 0.07 (−0.03, 0.17) | 0.15 | 0.10 (−0.01, 0.21) | 0.09 | −0.07 (−0.19, 0.04) | 0.21 |
| HDL-C (mmol/L) | ||||||
| Age < 60 years | 0.01 (−0.01, 0.03) | 0.27 | 0 (−0.03, 0.02) | 0.72 | 0.02 (−0.01, 0.04) | 0.13 |
| Age ≥ 60 years | 0.03 (0, 0.07) | 0.08 | 0.02 (−0.02, 0.06) | 0.35 | 0.03 (−0.01, 0.07) | 0.15 |
| LDL-C (mmol/L) | ||||||
| Age < 60 years | 0.01 (−0.04, 0.07) | 0.61 | 0.05 (−0.01, 0.11) | 0.14 | −0.06 (−0.13, 0.01) | 0.08 |
| Age ≥ 60 years | 0.06 (−0.05, 0.16) | 0.30 | 0.06 (−0.05, 0.18) | 0.28 | −0.03 (−0.16, 0.09) | 0.58 |
| Non-HDL-C (mmol/L) | ||||||
| Age < 60 years | 0.02 (−0.04, 0.08) | 0.42 | 0.08 (0.02, 0.15) | 0.01 | −0.04 (−0.12, 0.03) | 0.29 |
| Age ≥ 60 years | 0.04 (−0.06, 0.14) | 0.45 | 0.06 (−0.05, 0.18) | 0.27 | −0.09 (−0.21, 0.04) | 0.17 |
| TC:HDL-C | ||||||
| Age < 60 years | 0.02 (−0.04, 0.09) | 0.51 | 0.08 (0.01, 0.15) | 0.04 | −0.01 (−0.10, 0.07) | 0.75 |
| Age ≥ 60 years | −0.03 (−0.13, 0.08) | 0.64 | 0 (−0.12, 0.12) | 0.98 | −0.11 (−0.24, 0.01) | 0.08 |
| LDL-C:HDL-C | ||||||
| Age < 60 years | −0.01 (−0.05, 0.04) | 0.81 | 0.04 (−0.01, 0.09) | 0.12 | −0.07 (−0.13, −0.01) | 0.03 |
| Age ≥ 60 years | 0.01 (−0.08, 0.10) | 0.90 | 0.02 (−0.08, 0.12) | 0.71 | −0.05 (−0.15, 0.06) | 0.36 |
| Lipoprotein (a) (mg/L) | ||||||
| Age < 60 years | 1.59 (−7.41, 10.58) | 0.73 | 4.72 (−4.88, 14.33) | 0.34 | 0.05 (−11.30, 11.40) | 0.99 |
| Age ≥ 60 years | 5.40 (−10.92, 21.72) | 0.52 | 9.87 (−8.46, 28.20) | 0.29 | −2.98 (−22.24, 16.29) | 0.76 |
| Glucose homeostasis biomarkers | ||||||
| Glucose (mmol/L) | ||||||
| Age < 60 years | −0.02 (−0.09, 0.05) | 0.60 | −0.04 (−0.11, 0.04) | 0.34 | 0.03 (−0.05, 0.12) | 0.47 |
| Age ≥ 60 years | −0.02 (−0.16, 0.11) | 0.74 | −0.10 (−0.26, 0.05) | 0.18 | −0.13 (−0.29, 0.03) | 0.10 |
| Insulin (μIU/mL) | ||||||
| Age < 60 years | 0.18 (−0.80, 1.17) | 0.71 | −0.12 (−1.17, 0.94) | 0.83 | 0.84 (−0.41, 2.08) | 0.19 |
| Age ≥ 60 years | 0.91 (−1.75, 3.57) | 0.50 | 0.42 (−2.56, 3.39) | 0.78 | −0.58 (−3.69, 2.54) | 0.72 |
| HOMA-IR | ||||||
| Age < 60 years | −0.04 (−0.33, 0.26) | 0.81 | −0.16 (−0.48, 0.16) | 0.32 | 0.13 (−0.25, 0.51) | 0.50 |
| Age ≥ 60 years | 0.10 (−0.75, 0.96) | 0.82 | −0.08 (−1.04, 0.87) | 0.87 | −0.45 (−1.45, 0.55) | 0.37 |
| HbA1c (%) | ||||||
| Age < 60 years | 0.05 (0, 0.09) | <0.05 | 0 (−0.05, 0.05) | 0.97 | 0.09 (0.03, 0.15) | <0.01 |
| Age ≥ 60 years | −0.05 (−0.13, 0.04) | 0.26 | −0.17 (−0.27, −0.08) | <0.01 | 0.03 (−0.07, 0.13) | 0.57 |
| hsCRP (mg/L) | ||||||
| Age < 60 years | −0.12 (−0.48, 0.24) | 0.50 | −0.27 (−0.65, 0.12) | 0.18 | 0.27 (−0.19, 0.72) | 0.25 |
| Age ≥ 60 years | −0.46 (−1.34, 0.42) | 0.30 | −0.26 (−1.24, 0.72) | 0.60 | −0.86 (−1.89, 0.17) | 0.10 |
| Uric acid (μmol/L) | ||||||
| Age < 60 years | 7.62 (2.64, 12.61) | <0.01 | 15.55 (10.21, 20.89) | <0.01 | −4.81 (−11.09, 1.48) | 0.13 |
| Age ≥ 60 years | 7.76 (−0.91, 16.43) | 0.08 | 15.21 (5.54, 24.88) | <0.01 | −5.43 (−15.57, 4.72) | 0.29 |
| CMRF | Total Protein | Animal Protein | Plant Protein | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Lipid and lipoprotein profiles | ||||||
| TG (mmol/L) | ||||||
| Northern | 0.02 (−0.09, 0.14) | 0.71 | 0.09 (−0.03, 0.22) | 0.14 | −0.03 (−0.15, 0.09) | 0.59 |
| Southern | 0.01 (−0.06, 0.09) | 0.75 | 0.04 (−0.04, 0.12) | 0.35 | 0.04 (−0.07, 0.14) | 0.52 |
| TC (mmol/L) | ||||||
| Northern | 0.12 (0.04, 0.21) | <0.01 | 0.14 (0.05, 0.24) | <0.01 | −0.01 (−0.10, 0.08) | 0.87 |
| Southern | 0.05 (−0.01, 0.12) | 0.08 | 0.10 (0.03, 0.17) | <0.01 | 0.01 (−0.08, 0.09) | 0.90 |
| HDL-C (mmol/L) | ||||||
| Northern | 0.02 (−0.01, 0.05) | 0.15 | 0.02 (−0.01, 0.05) | 0.26 | 0.01 (−0.02, 0.04) | 0.63 |
| Southern | 0.01 (−0.01, 0.03) | 0.35 | 0.00 (−0.03, 0.02) | 0.90 | 0.04 (0.01, 0.07) | 0.01 |
| LDL-C (mmol/L) | ||||||
| Northern | 0.09 (−0.01, 0.18) | 0.06 | 0.06 (−0.03, 0.16) | 0.20 | 0.00 (−0.09, 0.10) | 0.95 |
| Southern | 0.02 (−0.04, 0.08) | 0.48 | 0.07 (0, 0.13) | 0.04 | −0.07 (−0.15, 0.01) | 0.11 |
| Non-HDL-C (mmol/L) | ||||||
| Northern | 0.08 (−0.01, 0.18) | 0.08 | 0.11 (0.01, 0.21) | 0.04 | −0.02 (−0.11, 0.08) | 0.75 |
| Southern | 0.04 (−0.02, 0.11) | 0.19 | 0.10 (0.03, 0.17) | <0.01 | −0.03 (−0.12, 0.06) | 0.46 |
| TC:HDL-C | ||||||
| Northern | 0.06 (−0.05, 0.17) | 0.28 | 0.07 (−0.05, 0.19) | 0.27 | 0.01 (−0.10, 0.12) | 0.84 |
| Southern | 0.02 (−0.05, 0.09) | 0.57 | 0.06 (−0.01, 0.14) | 0.10 | −0.05 (−0.14, 0.05) | 0.36 |
| LDL-C:HDL-C | ||||||
| Northern | 0.04 (−0.04, 0.12) | 0.30 | 0.02 (−0.06, 0.11) | 0.60 | 0.02 (−0.07, 0.10) | 0.71 |
| Southern | 0 (−0.05, 0.05) | 0.91 | 0.05 (−0.01, 0.10) | 0.09 | −0.10 (−0.18, −0.03) | <0.01 |
| Lipoprotein (a) (mg/L) | ||||||
| Northern | 1.50 (−12.03, 15.04) | 0.83 | 0.69 (−14.15, 15.53) | 0.93 | −3.06 (−17.06, 10.93) | 0.67 |
| Southern | 2.69 (−7.21, 12.60) | 0.59 | 8.26 (−2.45, 18.97) | 0.13 | −2.05 (−15.96, 11.86) | 0.77 |
| Glucose homeostasis biomarkers | ||||||
| Glucose (mmol/L) | ||||||
| Northern | −0.08 (−0.20, 0.03) | 0.17 | −0.18 (−0.31, −0.05) | <0.01 | −0.06 (−0.19, 0.06) | 0.30 |
| Southern | 0.03 (−0.05, 0.10) | 0.47 | 0.02 (−0.06, 0.09) | 0.71 | 0.06 (−0.04, 0.16) | 0.25 |
| Insulin (μIU/mL) | ||||||
| Northern | 0.80 (−0.87, 2.47) | 0.35 | 0.17 (−1.66, 1.99) | 0.86 | 0.32 (−1.41, 2.05) | 0.72 |
| Southern | 0.07 (−1.21, 1.36) | 0.91 | −0.42 (−1.82, 0.97) | 0.55 | 0.95 (−0.84, 2.75) | 0.30 |
| HOMA-IR | ||||||
| Northern | 0.11 (−0.48, 0.70) | 0.71 | −0.17 (−0.81, 0.48) | 0.61 | −0.10 (−0.71, 0.51) | 0.75 |
| Southern | −0.07 (−0.43, 0.29) | 0.71 | −0.23 (−0.62, 0.16) | 0.25 | 0.18 (−0.33, 0.68) | 0.50 |
| HbA1c (%) | ||||||
| Northern | −0.05 (−0.12, 0.02) | 0.19 | −0.16 (−0.23, −0.08) | <0.01 | −0.02 (−0.09, 0.06) | 0.65 |
| Southern | 0.06 (0.02, 0.11) | <0.01 | 0.03 (−0.03, 0.08) | 0.33 | 0.17 (0.10, 0.23) | <0.01 |
| hsCRP (mg/L) | ||||||
| Northern | 0.04 (−0.39, 0.48) | 0.84 | −0.02 (−0.50, 0.46) | 0.95 | −0.07 (−0.52, 0.39) | 0.77 |
| Southern | −0.33 (−0.83, 0.18) | 0.20 | −0.40 (−0.95, 0.15) | 0.15 | −0.03 (−0.74, 0.68) | 0.94 |
| Uric acid (μmol/L) | ||||||
| Northern | 6.31 (−1.38, 14.00) | 0.11 | 21.38 (13.01, 29.75) | <0.01 | −9.23 (−17.16, −1.30) | 0.02 |
| Southern | 10.78 (5.25, 16.30) | <0.01 | 14.08 (8.07, 20.09) | <0.01 | 1.82 (−5.89, 9.53) | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Cui, Z.; Li, M.; Li, T.; Wu, F.; Kang, T.; Meng, H. Associations between Dietary Animal and Plant Protein Intake and Cardiometabolic Risk Factors—A Cross-Sectional Study in China Health and Nutrition Survey. Nutrients 2021, 13, 336. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020336
Meng S, Cui Z, Li M, Li T, Wu F, Kang T, Meng H. Associations between Dietary Animal and Plant Protein Intake and Cardiometabolic Risk Factors—A Cross-Sectional Study in China Health and Nutrition Survey. Nutrients. 2021; 13(2):336. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020336
Chicago/Turabian StyleMeng, Shuangli, Zhixin Cui, Minjuan Li, Ting Li, Feng Wu, Tong Kang, and Huicui Meng. 2021. "Associations between Dietary Animal and Plant Protein Intake and Cardiometabolic Risk Factors—A Cross-Sectional Study in China Health and Nutrition Survey" Nutrients 13, no. 2: 336. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020336