Fabrication and Application of SERS-Active Cellulose Fibers Regenerated from Waste Resource
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Au Colloid
2.3. Fabrication of Regenerated Cellulose Fiber-Au Composite
2.4. Apparatus
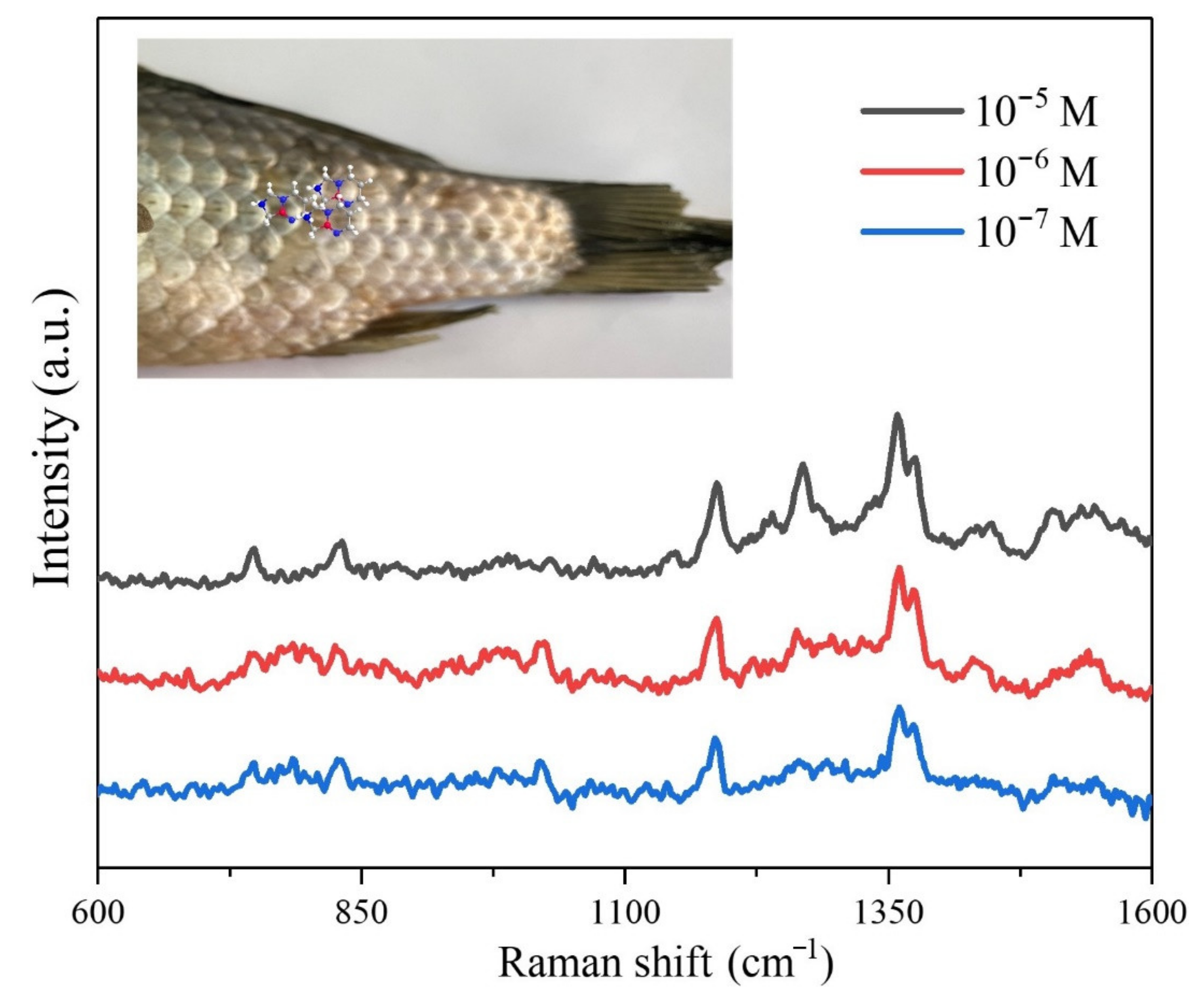
2.5. Detection of Dimetridazole Using the Regenerated Cellulose Fiber-Au
3. Results and Discussion
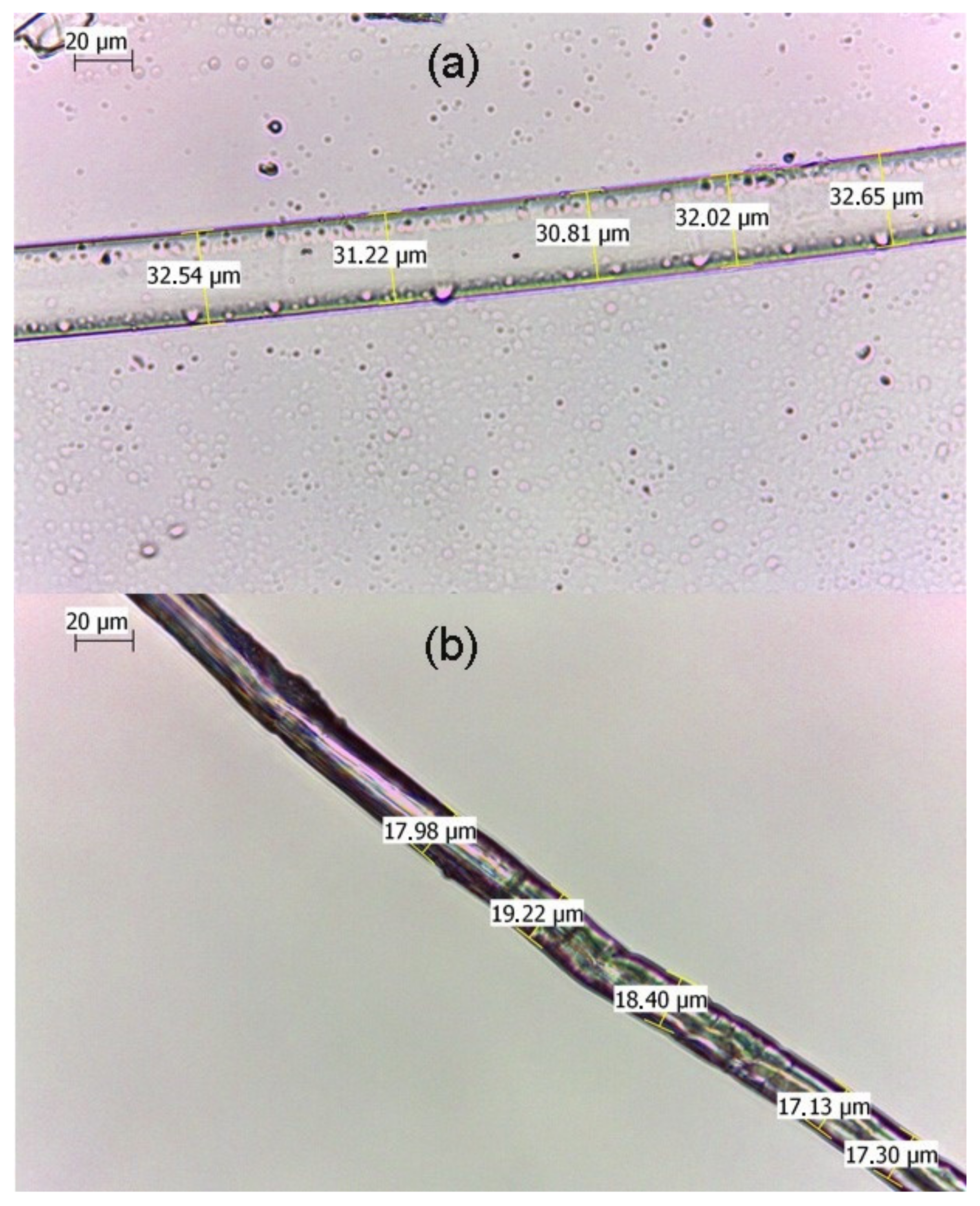
3.1. Preparation of Regenerated Cellulose Fiber-Au NPs Composites
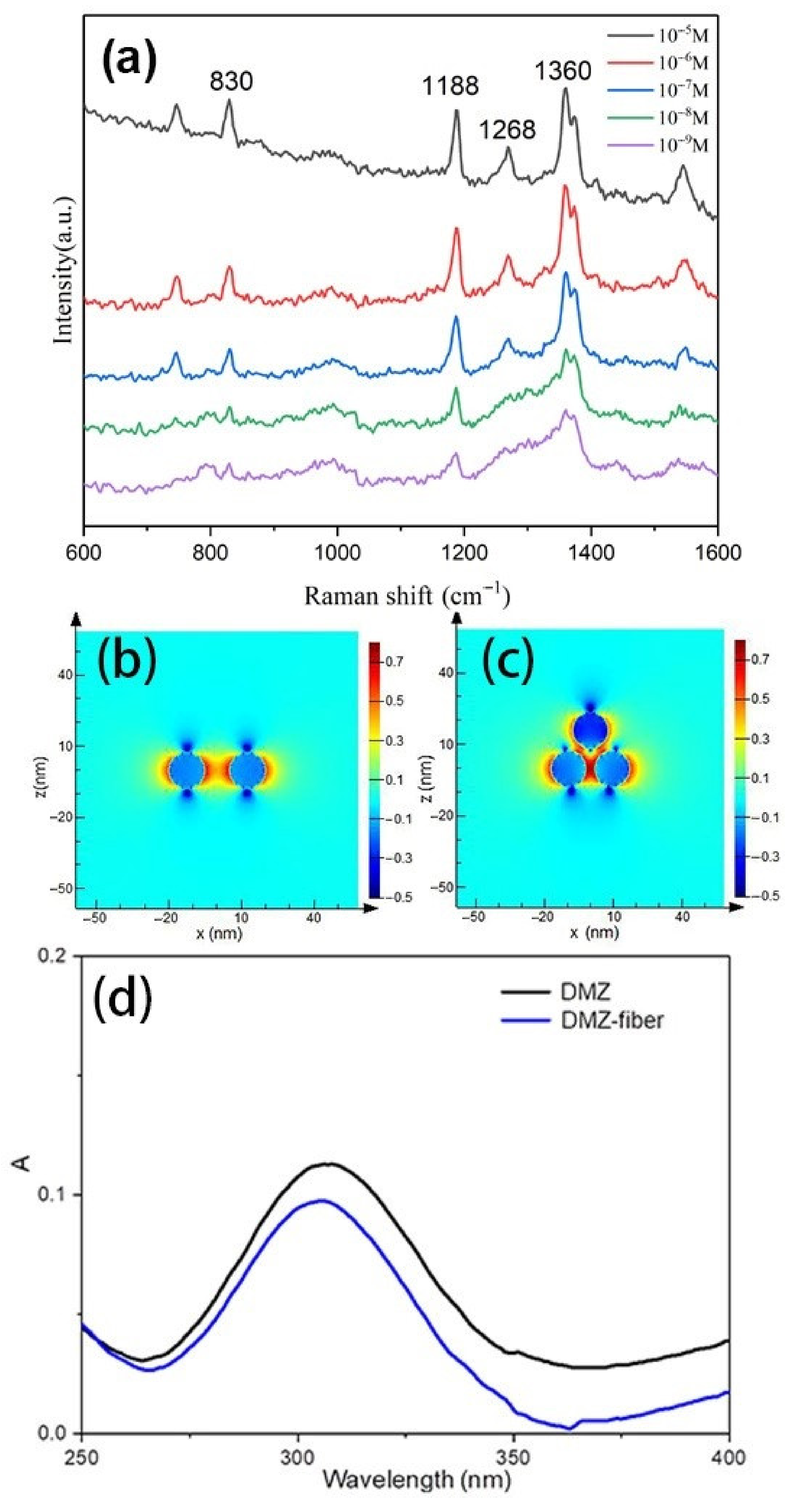
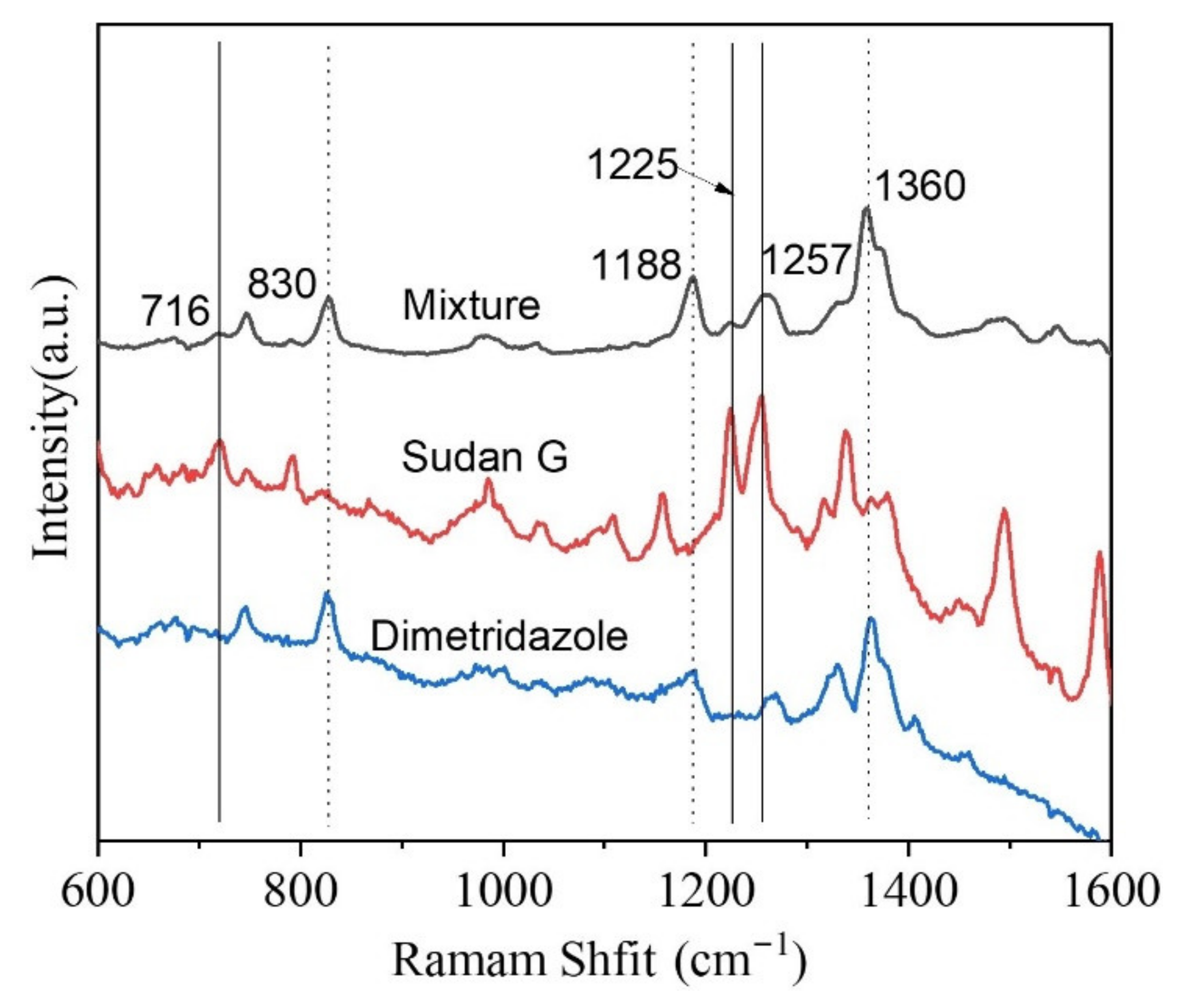
3.2. SERS Application of Regenerated Cellulose Fiber-Au Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Zhang, L.; Zeng, Q.; Liu, X.; Lai, W.-Y.; Zhang, L. Cellulose microcrystals with brush-like architectures as flexible all-solid-state polymer electrolyte for lithium-ion battery. ACS Sustain. Chem. Eng. 2020, 8, 3200–3207. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Ma, Y.; Asaadi, S.; Johansson, L.S.; Ahvenainen, P.; Reza, M.; Alekhina, M.; Rautkari, L.; Michud, A.; Hauru, L.; Hummel, M. High-strength composite fibers from cellulose–lignin blends regenerated from ionic liquid solution. ChemSusChem 2015, 8, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.; Guo, J.; Yang, F.; Zhu, Y.; Song, J.; Jin, Y.; Rojas, O.J. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: Adsorption behavior and mechanism. J. Hazard. Mater. 2021, 403, 123666. [Google Scholar] [CrossRef]
- Chitpong, N.; Husson, S.M. Polyacid functionalized cellulose nanofiber membranes for removal of heavy metals from impaired waters. J. Membr. Sci. 2017, 523, 418–429. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Y.; Lu, A.; Fu, Q.; Hu, J.; Zhang, L. Cellulose/chitosan composite multifilament fibers with two-switch shape memory performance. ACS Sustain. Chem. Eng. 2019, 7, 6981–6990. [Google Scholar] [CrossRef]
- Aka, E.C.; Nongbe, M.C.; Ekou, T.; Ekou, L.; Coeffard, V.; Felpin, F.-X. A fully bio-sourced adsorbent of heavy metals in water fabricated by immobilization of quinine on cellulose paper. J. Environ. Sci. 2019, 84, 174–183. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Li, J. Flexible, highly conductive, and free-standing reduced graphene oxide/polypyrrole/cellulose hybrid papers for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3819–3831. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Ma, L.; Xiang, C.; Li, L. TiO2-Doped chitosan microspheres supported on cellulose acetate fibers for adsorption and photocatalytic degradation of methyl orange. Polymers 2019, 11, 1293. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Mannan, A.; Hussain, I.; Hussain, I.; Zia, M. Effective removal of metal ions from aquous solution by silver and zinc nanoparticles functionalized cellulose: Isotherm, kinetics and statistical supposition of process. Environ. Nanotechnol. Monit. Manag. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Cui, R.; Ma, Y.; Yu, Q.; Kannegulla, A.; Wu, B.; Fan, H.; Wang, A.X.; Kong, X. Plasmonic cellulose textile fiber from waste paper for BPA sensing by SERS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117664. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Li, H.; Wang, H.; Wang, Z.; Zhou, W.; Liu, H. Preparation of cellulose fiber–TiO2 nanobelt–silver nanoparticle hierarchically structured hybrid paper and its photocatalytic and antibacterial properties. Chem. Eng. J. 2013, 228, 272–280. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Patil, A.; Meng, Z.; Liu, S.; Hou, C.; Zhang, Y.; Yu, W.; Guo, W.; Liu, X.Y. Graphene decorated carbonized cellulose fabric for physiological signal monitoring and energy harvesting. J. Mater. Chem. A 2020, 8, 12665–12673. [Google Scholar] [CrossRef]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Squire, K.; Tan, A.; Zhao, Y.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Trace detection of tetrahydrocannabinol in body fluid via surface-enhanced raman scattering and principal component analysis. ACS Sens. 2019, 4, 1109–1117. [Google Scholar] [CrossRef]
- Tian, X.; Zhai, P.; Guo, J.; Yu, Q.; Xu, L.; Yu, X.; Wang, R.; Kong, X. Fabrication of plasmonic cotton gauze-Ag composite as versatile SERS substrate for detection of pesticides residue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 257, 119766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, M.; Jiang, S.; Ding, F.; Wang, J. Coating fabrics with gold nanorods for colouring, UV-protection, and antibacterial functions. Nanoscale 2013, 5, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Zhang, C.; You, T.; Yang, N.; Gao, Y.; Jiang, L.; Yin, P. Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem. 2019, 287, 363–368. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Squire, K.; Kraai, J.A.; Tan, A.; Zhao, Y.; Rorrer, G.L.; Wang, A.X. Biological photonic crystal-enhanced plasmonic mesocapsules: Approaching single-molecule optofluidic-SERS sensing. Adv. Opt. Mater. 2019, 7, 1900415. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, C.; Xie, L.; Yuan, X.; Wei, X.; Huang, Q.; Chen, Y.; Lu, Y. Silver-Nanocellulose composite used as SERS substrate for detecting carbendazim. Nanomaterials 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Wang, X.; Huang, Y.; Lai, K.; Fan, Y. Rapid detection of trace methylene blue and malachite green in four fish tissues by ultra-sensitive surface-enhanced Raman spectroscopy coated with gold nanorods. Food Control 2019, 106, 106720. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Xu, L.; Wang, W.; Du, J.; Qu, M.; Han, X.; Yang, L.; Zhao, B. Ultrasensitive SERS detection of antitumor drug methotrexate based on modified Ag substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118589. [Google Scholar] [CrossRef]
- Qian, C.; Guo, Q.; Xu, M.; Yuan, Y.; Yao, J. Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film. RSC Adv. 2015, 5, 53306–53312. [Google Scholar] [CrossRef]
- Qu, L.-L.; Geng, Y.-Y.; Bao, Z.-N.; Riaz, S.; Li, H. Silver nanoparticles on cotton swabs for improved surface-enhanced Raman scattering, and its application to the detection of carbaryl. Microchim. Acta 2016, 183, 1307–1313. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, F.; Guang, S.; Cai, Z. Self-assembly of Ag nanoparticles on the woven cotton fabrics as mechanical flexible substrates for surface enhanced Raman scattering. J. Alloy. Compd. 2017, 726, 484–489. [Google Scholar] [CrossRef]
- Ma, Y.; Hummel, M.; Määttänen, M.; Särkilahti, A.; Harlin, A.; Sixta, H. Upcycling of waste paper and cardboard to textiles. Green Chem. 2016, 18, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Christma, F.L.A.; Toyin, A.J.; Gopinath, S.C.B.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, J.R.; Werts, M.H. Resonant light scattering spectroscopy of gold, silver and gold–silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Han, D.; Pang, Z.; Sun, Y.; Wang, Y.; Zhang, Y.; Yang, J.; Chen, L. Charge transfer in an ordered Ag/Cu2S/4-MBA system based on surface-enhanced raman scattering. J. Phys. Chem. C 2018, 122, 5599–5605. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Wang, Y.; Hua, T.; Guo, R.; Miao, D.; Jiang, S. Flexible, stable and sensitive surface-enhanced Raman scattering of graphite/titanium-cotton substrate for conformal rapid food safety detection. Cellulose 2020, 27, 941–954. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Guo, J.; Ma, Y.; Wang, A.X.; Kong, X.; Yu, Q. Fabrication and Application of SERS-Active Cellulose Fibers Regenerated from Waste Resource. Polymers 2021, 13, 2142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132142
Wang S, Guo J, Ma Y, Wang AX, Kong X, Yu Q. Fabrication and Application of SERS-Active Cellulose Fibers Regenerated from Waste Resource. Polymers. 2021; 13(13):2142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132142
Chicago/Turabian StyleWang, Shengjun, Jiaqi Guo, Yibo Ma, Alan X. Wang, Xianming Kong, and Qian Yu. 2021. "Fabrication and Application of SERS-Active Cellulose Fibers Regenerated from Waste Resource" Polymers 13, no. 13: 2142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132142





